Thoracoscopy is increasingly being used in the treatment of empyema. This study assesses feasibility, efficacy and safety in children.
Material and methodsClinical files of patients who underwent primary thoracoscopy for empyema between 2006 and 2014 were reviewed. Demographic, clinical and surgical data were analyzed and a comparison between the period before (period1) and after (period2) the learning curve was performed.
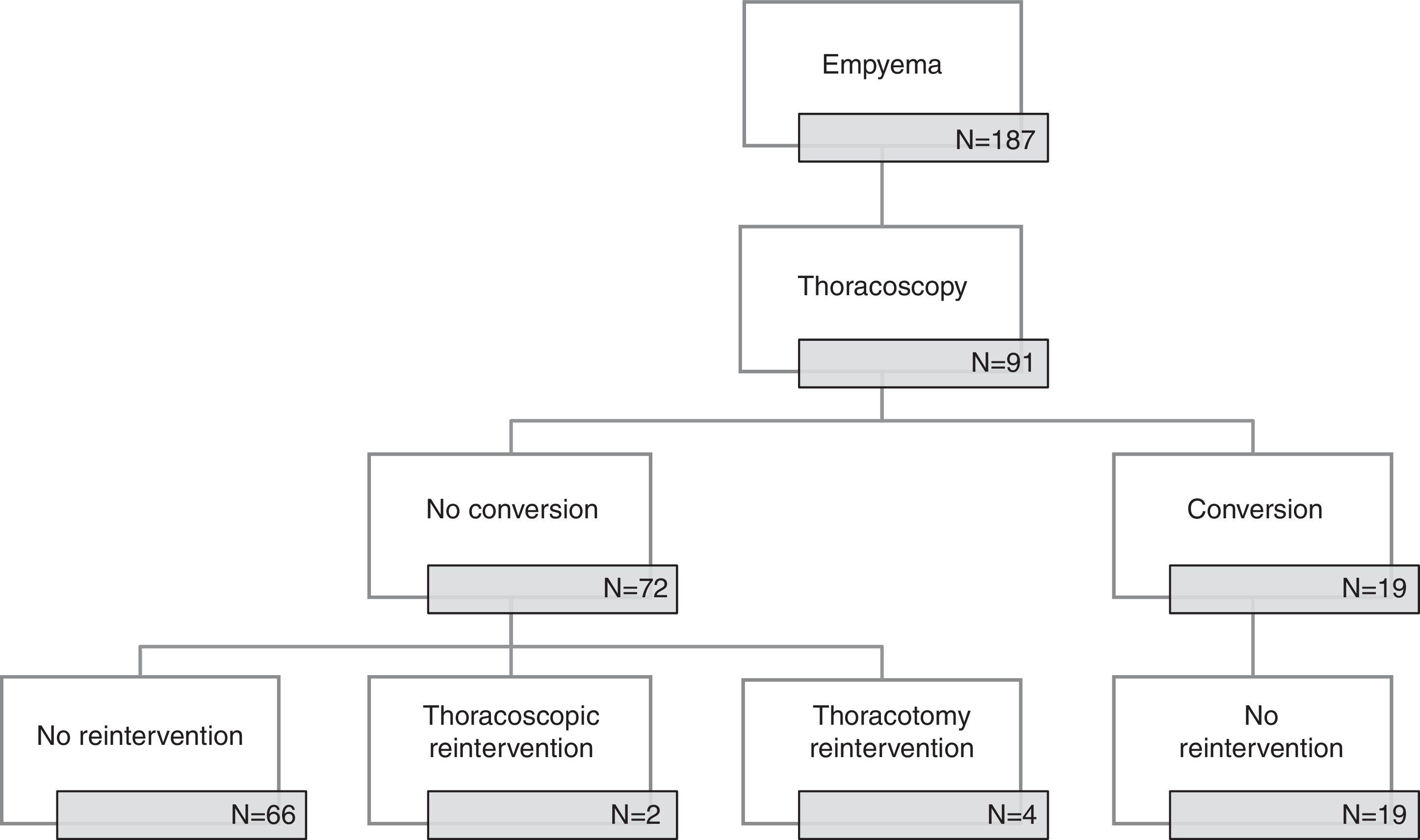
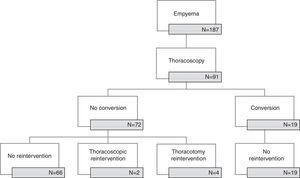
ResultsNinety-one patients (53 males, 58%) were submitted to thoracoscopy at a median age of 4 years. There were 19 conversions to thoracotomy with a steady decrease of conversion rate until 2009 (period1) and no conversions thereafter (period2). There was no difference in any of the analyzed parameters between patients submitted to thoracoscopy alone and those requiring conversion in period1. Six cases (6.6%) needed redo-operation (five in period2) and thoracotomy was the elected approach in four. Necrotizing pneumonia was present in 60% of the reoperated cases; in other words, in period2 3 out of 9 cases with necrotizing pneumonia required reintervention (p=0.07). Thoracotomy was avoided in sixty-eight (75%) patients (62% in period1 versus 92% in period2, p=0.001).
Discussion and conclusionsThoracoscopic approach for empyema is feasible and safe avoiding a significant number of thoracotomies after a short learning curve. An increase of reintervention rate should be expected, but throracoscopy alone is effective in the great majority of the cases. Necrotizing pneumonia may be associated with a higher risk of reintervention, as it is a contra-indication to thoracoscopy and probably surgery.
The incidence of empyema in children is increasing worldwide.1,2 Empyema occurs in nearly 1 in 150 children hospitalized with pneumonia, affecting about 3.3 per 100000 children.3,4 In contrast to their adult counterparts, children with empyema usually have a normal underlying lung and suppuration within the pleural cavity is most commonly a complication of acute bacterial pneumonia. Prognosis is excellent when appropriate treatment is administered early. Therapeutic options in children include systemic antibiotics, thoracocentesis, chest drain, fibrinolytic agents, and several surgical techniques such as thoracoscopy, video-assisted thoracoscopic surgery (VATS), mini-thoracotomy, and standard thoracotomy with lung decortication. No consensus has been reached about the optimal therapeutic strategy for pediatric empyema. A systematic review of randomized controlled trials (RCT) included three small RCTs comparing the outcomes of fibrinolytics and VATS with discordant findings: one study suggested better outcomes with primary operative treatment, but the other two found no significant difference in the measured outcomes except for the lowest cost of fibrinolytics.3,5 The most recent randomized multicenter clinical trial, concluded that drainage plus urokinase instillation is as effective as VATS as first-line treatment of septated empyema in children.6
Despite the enthusiasm around minimally invasive surgical approaches, conversion and reintervention during the thoracoscopic management of pediatric empyemas have not been profoundly studied as in adults literature.7
The aim of this work is to assess the feasibility, efficacy and safety of thoracoscopic approach in a series of pediatric empyema.
Materials and methodsStudy design and patient selectionAll pediatric patients (aged <18 years) admitted between January 2006 and December 2014 at Hospital São João in Porto, Portugal with empyema associated with community-acquired pneumonia were eligible. An empyema was defined as a loculated or septated effusion by imagiologic study or finding of pus or loculated effusion at the time of surgical intervention. Necrotizing pneumonia was defined as multiple small lucencies or cavities of non-enhancement on a contrast-enhanced chest computed tomography (CT). Patients submitted to non-operative treatment or primary thoracotomy were excluded; only patients who underwent primary thoracoscopic management were included in this study.
The following data were retrieved from the patient records: demography (age, sex, date of empyema diagnosis), clinical presentation (duration of preoperative fever, duration of preoperative empyema, pneumonia characteristics – such as necrotizing pneumonia, empyema location, intensive care unit (ICU) admission), preoperative investigations (analytical inflammatory markers, chest radiograph, chest ultrasound and chest computed tomography, the use of preoperative chest tube), antibiotic usage, operative details (duration of surgery, number of trocars used, intraoperative complications, conversion to open procedure) and outcomes (complications including hemorrhage, pneumatocele, bronchopleural fistula and postoperative need for mechanical ventilation, duration of postoperative fever, duration of postoperative chest tube, duration of hospital stay and mortality). Surgical delay was defined as the number of days from the diagnosis to thoracoscopic treatment.
The learning curve was defined as the period when conversion occurred (period1), the period thereafter was defined as period2.
Patients submitted to thoracoscopy alone were compared with those requiring conversion (during period1) regarding preoperative data, operative details and outcomes. Patients needing reoperation during period2 were analyzed in a search for possible predictors of reintervention.
Globally, patients with necrotizing pneumonia were compared with those without this diagnosis regarding the preoperative data, operative details and outcomes.
Surgical techniquesPatients were submitted to thoracoscopy using either two or three trocars; in case of conversion or reoperation the posterolateral thoracotomy approach was used. At the completion of the procedure one or two chest tubes were inserted under thoracoscopic visualization and removed when drainage was minimal. Patients were discharged after chest tube removal and completion of intravenous antibiotics.
Statistical methodsStatistical analysis was conducted using SPSS® software, version 22 and all reported p values are two-tailed, statistical significance set at 5%. Frequencies were compared with Fisher's exact test for categoric variables, and continuous variables were compared using the t test and analysis of variance.
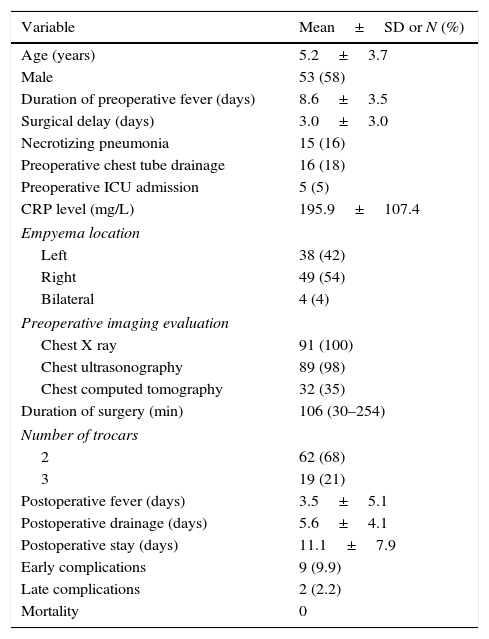
ResultsFrom January 2006 to December 2014, 91 out of 187 pediatric patients with empyema in our institution were submitted to thoracoscopic management (Fig. 1). There were 53 boys (58.2%) and 38 girls (41.8%) with an age of 5.2±3.6 years. Fifty patients (55%) were referred from other hospitals. Clinical, radiologic and surgical data are presented in Table 1.
Clinical and surgical data.
| Variable | Mean±SD or N (%) |
|---|---|
| Age (years) | 5.2±3.7 |
| Male | 53 (58) |
| Duration of preoperative fever (days) | 8.6±3.5 |
| Surgical delay (days) | 3.0±3.0 |
| Necrotizing pneumonia | 15 (16) |
| Preoperative chest tube drainage | 16 (18) |
| Preoperative ICU admission | 5 (5) |
| CRP level (mg/L) | 195.9±107.4 |
| Empyema location | |
| Left | 38 (42) |
| Right | 49 (54) |
| Bilateral | 4 (4) |
| Preoperative imaging evaluation | |
| Chest X ray | 91 (100) |
| Chest ultrasonography | 89 (98) |
| Chest computed tomography | 32 (35) |
| Duration of surgery (min) | 106 (30–254) |
| Number of trocars | |
| 2 | 62 (68) |
| 3 | 19 (21) |
| Postoperative fever (days) | 3.5±5.1 |
| Postoperative drainage (days) | 5.6±4.1 |
| Postoperative stay (days) | 11.1±7.9 |
| Early complications | 9 (9.9) |
| Late complications | 2 (2.2) |
| Mortality | 0 |
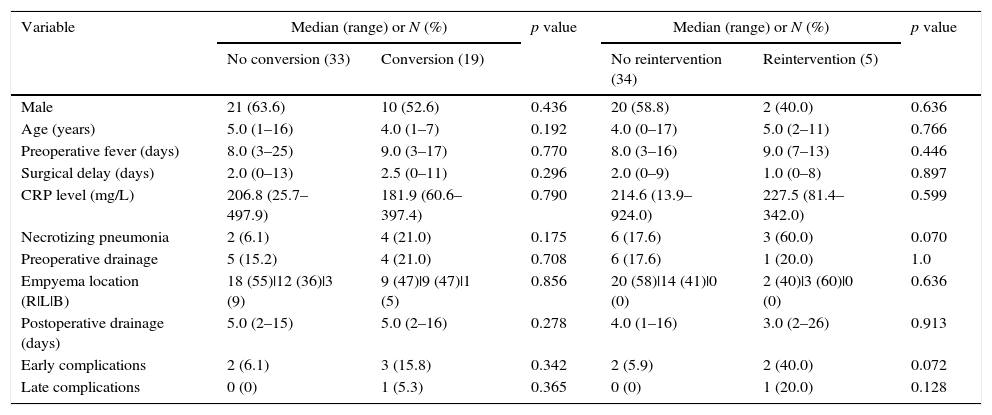
Period1 extended until 2009 and there were 19 (56%) conversions to thoracotomy with a steady decrease in the conversion rate; in the period2 from 2010 to 2014 there were no conversions. The comparison between converted and non-converted cases during period1 is presented in Table 2. The most frequent cause of conversion was organized empyema with thick fibrin peel in 14 patients, followed by necrotizing pneumonia with fistula (3), bleeding (1) and diaphragmatic perforation (1).
Comparative data of converted and non-converted patients (period1) and intervened and non-intervened patients (period2).
| Variable | Median (range) or N (%) | p value | Median (range) or N (%) | p value | ||
|---|---|---|---|---|---|---|
| No conversion (33) | Conversion (19) | No reintervention (34) | Reintervention (5) | |||
| Male | 21 (63.6) | 10 (52.6) | 0.436 | 20 (58.8) | 2 (40.0) | 0.636 |
| Age (years) | 5.0 (1–16) | 4.0 (1–7) | 0.192 | 4.0 (0–17) | 5.0 (2–11) | 0.766 |
| Preoperative fever (days) | 8.0 (3–25) | 9.0 (3–17) | 0.770 | 8.0 (3–16) | 9.0 (7–13) | 0.446 |
| Surgical delay (days) | 2.0 (0–13) | 2.5 (0–11) | 0.296 | 2.0 (0–9) | 1.0 (0–8) | 0.897 |
| CRP level (mg/L) | 206.8 (25.7–497.9) | 181.9 (60.6–397.4) | 0.790 | 214.6 (13.9–924.0) | 227.5 (81.4–342.0) | 0.599 |
| Necrotizing pneumonia | 2 (6.1) | 4 (21.0) | 0.175 | 6 (17.6) | 3 (60.0) | 0.070 |
| Preoperative drainage | 5 (15.2) | 4 (21.0) | 0.708 | 6 (17.6) | 1 (20.0) | 1.0 |
| Empyema location (R|L|B) | 18 (55)|12 (36)|3 (9) | 9 (47)|9 (47)|1 (5) | 0.856 | 20 (58)|14 (41)|0 (0) | 2 (40)|3 (60)|0 (0) | 0.636 |
| Postoperative drainage (days) | 5.0 (2–15) | 5.0 (2–16) | 0.278 | 4.0 (1–16) | 3.0 (2–26) | 0.913 |
| Early complications | 2 (6.1) | 3 (15.8) | 0.342 | 2 (5.9) | 2 (40.0) | 0.072 |
| Late complications | 0 (0) | 1 (5.3) | 0.365 | 0 (0) | 1 (20.0) | 0.128 |
(R|L|B) – (Right | Left | Bilateral).
Overall reintervention rate was 6.6% (6 cases) and thoracothomy was the elected approach for reoperation in four cases. None of the converted cases was reoperated. There was a higher reintervention rate in period2 than in period1 (1/52, 1.9% vs. 5/39, 12.8%, p=0.08). Fistula was the most common cause of reintervention and was associated with pneumonia (3 cases) or pneumatocele (1 case); relapse was the cause of reoperation in the remaining two cases. Necrotizing pneumonia was present in 60% of the reoperated cases, in other word, in period2 3 out of 9 cases with necrotizing pneumonia required reintervention (p=0.07).
Thoracoscopic approach spared 68 patients (75%) from thoracotomy; a higher proportion of patients were spared after 2009 (62% period1 vs. 92% period2, p<0.001).
There were no recurrences after hospital discharge, with a follow-up ranging from 1 to 27 months (median, 6 months).
Globally, patients with necrotizing pneumonia were identical regarding all clinical data, except for longer overall length of stay (median 20 versus 12, p=0.004) and longer post-operative length of stay (17 versus 8, p=0.002).
DiscussionThe optimal management of pediatric empyema remains controversial.8 Thoracocentesis and antibiotics alone have low success rate (6–20%) in the treatment of empyema, even in early stage disease. The role of thoracoscopy as compared to fibrinolysis in pediatric empyema is still controversial: studies comparing the effectiveness of fibrinolytics versus thoracoscopy suggest a higher rate of primary treatment failure in the fibrinolytics approach;9–11 however, a recent multicenter RCT concluded that fibrinolytics instillation is as effective as video-assisted thoracoscopic surgery for primary treatment of pediatric septated empyema.6 Our institution approach has been in favor of early surgical intervention following the IPEG guidelines;12 as the placement of a chest tube in such young patients requires anesthesia, this timing is used for a diagnostic thoracoscopy and proceeding with the treatment.12
Conversion rates from thoracoscopy to thoracotomy are quite different among studies, ranging from 5.6% to 61%.7 In our study, the overall conversion rate was 21%, however it is worth noting that after the introduction of thoracoscopy in our institution, thoracoscopy was always the first-line approach independently of the stage of the empyema and that might have resulted in higher conversion and reintervention rates.
Additionally, there was a significant reduction of the conversion rate in the first four years (period1). As patients requiring conversion during this period were no different from those submitted to thoracoscopy alone, this reduction could be fully explained by a learning curve associated with the increased surgical expertise. There was no increased morbidity or mortality associated with conversion, meaning that the thoracoscopic learning curve is safe with no detriment for the patients. Our results concur with previous series reporting postoperative fever lasting 2–5 days, duration of postoperative chest tube of 4–6 days, median postoperative stay of 6–13 days and total hospital stay of 8–21 days. 9,13 Therefore, pediatric surgeons with expertise in open approach should be encouraged to progressively step into minimally invasive surgical techniques embracing their advantages without fearing an increased morbidity. After the learning curve, there was no conversion but an increase in reintervention rate was identified; nevertheless thoracoscopic approach means the great majority (92%) of patients are spared thoracotomy. The parallel increase in the reintervention rate along with decrease in conversion rate may indicate that a subset of patients ultimately needs a thoracotomy; however, our sample was too small to confirm this statement.
Early surgical intervention has already been associated with better outcomes such as simpler procedures, shorter hospitalization, shorter intravenous antibiotic therapy, less postoperative complications and more important lower failure of the thoracoscopic approach in both adult and pediatric patients.4,7,12,14–18 In our series, preoperative fever duration was not related either to reintervention or conversion rates. The fever was the only studied symptom and on average our patients were operated eight days after the onset of this symptom, with a short surgical delay: in median thoracoscopy was performed two days after diagnosis. In the literature, a great importance is given to early transfer and prompt surgery to achieve more favorable outcomes, reducing the morbidity and mortality.17–20 Previous studies have recommended the surgical intervention not delayed beyond 4–7 days.18,19
Apart from the comparison studies between early and delayed interventions, one study analyzed CT scan features from adult empyema (amount of pleural fluid, type of effusion, number of loculations, pleural fluid characteristics and pleural thickening) and concluded that patients presenting with fever and pleural thickening and who are operated on more than 20 days after the onset of symptoms are likely to require a thoracotomy for appropriate treatment.7 CT scan data could be important in predicting either conversion or reintervension, unfortunately we are unable to obtain CT scan data from all patients, as this exam is not routinely performed in the pediatric population because of its associated radiation. Nevertheless, our data suggest a relationship between necrotizing pneumonia on CT scan and reintervention. Moreover necrotizing pneumonia was associated with increased length of hospital stay.
The radiation exposure imposes a challenge in the diagnosis of necrotizing pneumonia in the pediatric population. In result, pulmonary necrosis is probably under-diagnosed during the preoperative evaluation of empyema; it is of utmost importance to establish the difference between empyema and pulmonary necrosis, as this interferes in the treatment and outcome.21,22 The risk and benefits of the CT scan should be evaluated or in alternative, a magnetic resonance imaging (MRI) can be performed.
Conclusions and limitationsIn conclusion, thoracoscopic approach for empyema is feasible and safe avoiding a significant number of thoracotomies after a rapid learning curve. An increase in the reintervention rate should be expected thereafter, but thoracoscopy alone is effective in the great majority of the cases.
Necrotizing pneumonia in the CT scan is possibly associated with reintervention. Beside the retrospective nature of the study, the major limitation was the mentioned lack of descriptors of empyema, as ultrasound descriptions are not standardized and CT scan is only ordered in few pediatric patients. In the future, preoperative ultrasound or MRI features should also be considered in a larger prospective study in order to determine predictors of failure of thoracoscopic approach.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.