The management and treatment of Chronic Obstructive Pulmonary Disease (COPD) are based on a cutoff point either of ≥ 10 on the COPD Assessment Test (CAT) or of ≥ 2 of the Medical Research Council (mMRC). Up to now, no study has assessed the equivalence between CAT and mMRC, as related to exercise tolerance in COPD. The aim of this study was to investigate as primary outcome the relationship between CAT and mMRC and maximal exercise capacity in COPD patients. We also evaluated as secondary outcome the agreement between CAT (≥ 10) and mMRC (≥ 2) to categorize patients according to their exercise tolerance.
Material and methods118 consecutive COPD patients (39 females), aged between 47 and 85 years with a wide range of airflow obstruction and lung hyperinflation were studied. Maximal exercise capacity was assessed by cardiopulmonary exercise test.
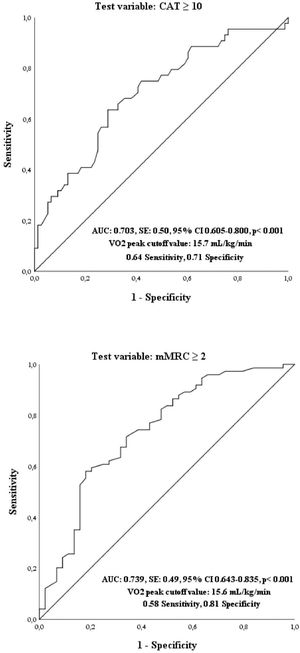
ResultsCAT and mMRC scores were significantly related to VO2 peak (p<0.01). CAT (≥ 10) and mMRC (≥ 2) have a high likelihood to be associated to a value of VO2 peak less than 15.7 and 15.6 mL/kg/min, respectively. The interrater agreement between CAT (≥ 10) and mMRC (≥ 2) was found to be fair (κ = 0.20) in all patients but slight when they were subdivided in those with VO2 peak < 15 mL/kg/min and in those with VO2 peak ≥ 15 mL/kg/min (κ = 0.10 and κ = 0.20 respectively).
ConclusionThis study shows that CAT and mMRC are useful tools to predict exercise tolerance in COPD, but they cannot be considered as supplementary measures.
Chronic Obstructive Pulmonary Disease (COPD) is an inflammatory disease of the lungs characterized by chronic, progressive and not fully reversible airflow limitation.1 COPD is primarily a lung disease, but can induce systemic effects with a significant impairment in exercise tolerance, health status and patient quality of life1 and is now considered as a heterogeneous disease with multiple phenotypes and endotypes.2 Accordingly, COPD management and treatment imply not only spirometric evaluation, but also a multidimensional assessment of the functional status and patients’ quality of life.1 In this context, it is worth noting that more than half of COPD patients complain of fatigue which may have a substantial impact on physical activity, quality of life, hospitalization rate, morbidity and mortality.3
The COPD Assessment Test (CAT) is a self-administered questionnaire consisting of eight items, which evaluate the most burdensome symptoms and limitations of the patients.4 The score for each item ranges from 0 to 5 and the total score (0–40) provides a simple and quantified measure of health-related quality of life, with higher scores indicating poorer health status.4 There is a body of evidence concerning the reliability of CAT as predictor of diagnosis,5,6 disease exacerbation,6-8 and mortality.9 On the other hand, a simple measure of dyspnea, such as the modified Medical Research Council (mMRC) scale is also considered appropriate for the assessment of the symptoms,10 since it relates to health status,11 and may predict mortality risk of COPD patients.12 According to the Global Initiative for COPD (GOLD) document, symptom assessment and treatment of the patients are based on a cutoff point of ≥ 10 on the CAT, which is considered equivalent to that of ≥ 2 of the mMRC scale.1
Up to now there has been limited evidence specifically addressed to evaluate the relationship between CAT, mMRC and maximal exercise capacity in COPD patients.13-15 Furthermore, so far no study has assessed the equivalence between CAT and mMRC, as related to exercise capacity in COPD. It is worth noting that both performance during standardized exercise tests and their related pathophysiological responses are recognized as important biomarkers of the multidimensional assessment of cardiac and pulmonary diseases.16 In a small sample of COPD patients, CAT score together with the Forced Expiratory Volume at 1st second (FEV1) value was found to predict oxygen uptake in COPD.13 In COPD patients with airflow obstruction degree ranging from mild to severe, mMRC dyspnea scale score was weakly and negatively correlated with the peak oxygen uptake.14 In addition, in COPD patients with the same severity of airflow obstruction, a high score of mMRC was related to a poor maximum exercise capacity.15
The aim of the present study was, therefore, as primary outcome to investigate in a large cohort of COPD patients, the relationship between CAT and mMRC and the maximal exercise capacity assessed by means of the cardiopulmonary exercise test (CPET). Furthermore, we evaluated as secondary outcome the agreement between CAT (≥ 10) and mMRC (≥ 2) in order to categorize COPD patients according to their maximal exercise capacity.
Material and methodsSubjectsOutpatients with COPD, diagnosed according to the GOLD criteria,1 were consecutively enrolled in the study from December 2018 to January 2020. The inclusion criteria were: smoking history of ≥10 pack-years; post bronchodilator forced expiratory volume at 1st second (FEV1)/forced vital capacity (FVC) <0.7; regular pharmacological treatment over the previous 6 months. The exclusion criteria were: a COPD exacerbation in the previous 2 months; patients with the coexistence of another chronic pulmonary disease; patients with severe comorbidities associated to COPD (i.e. unstable cardiovascular disease or cancer); patients unable to perform all the tests required.
Patients characteristics were recorded at baseline: anthropometric variables (age, sex and body mass index – BMI, in kg/m2), smoking habit, CAT score (Italian version),4 rate of COPD exacerbations and domiciliary medications. The Italian version of the five-point mMRC scale was used to assess the daily living activity-related dyspnea.10
Based on the GOLD document,1 COPD patients are considered at increased risk, when their CAT and nMRC values are greater than 10 and 2, respectively. Accordingly, we subdivided the patients into two subgroups, by choosing the cutoff points of ≥ 10 for the CAT and of ≥ 2 for the mMRC scale.
The study protocol was approved by the local Ethics Committee (approval number n. 14,718). All patients gave their informed consent.
Pulmonary function testsPulmonary function tests were performed with a flow-sensing spirometer and a body plethysmograph (Vmax 22 and 6200; SensorMedics, Yorba Linda, CA, USA). FEV1, and FVC were recorded and reported as absolute values (L) and percentage of predicted value (% pred); FEV1/FVC were expressed as a ratio and taken as index of airway obstruction.
Body plethysmography was used to quantify the thoracic gas volume (TGV) and total lung capacity (TLC,% pred) was calculated by adding TGV to inspiratory capacity (IC). The residual Volume/TLC ratio was taken as an index of static hyperinflation.
Lung transfer factor for carbon monoxide (TLco,% pred) was assessed via the single breath method using a mixture of carbon monoxide and methane.
Patients were forbidden to use bronchodilators 12 h before baseline spirometry. The reversibility test was carried out by second spirometry 15 min after inhalation of salbutamol 400 μg.
CPETA cycloergometer (Corival PB, Lode BV, Groningen, The Netherlands) was used to carried out CPET, in agreement with the current standardized procedure.17 During the test patients were continuously monitored by a 12-lead electrocardiogram (ECG, CardioPerfect, Welch Allyn, Delft, The Netherlands) and a pulse oximeter (Pulse Oximeter 8600, Nonin Medical Inc, MPLS, Mn U.S.). The exercise protocol included: 3 min of rest, a further 3 min of unloaded cycling, followed by a progressive increment of 5–15 W each minute, depending on the anthropometric characteristics of patients and individual functional impairment. The Wasserman's equation was used to calculate the work rate increment.18 Blood pressure (mm Hg) was measured every 2 min. Stopping criteria included: unsustainable dyspnea, muscular fatigue, chest pain, significant ECG ST-segment depression, a drop in systolic blood pressure or arterial oxygen saturation <84%.
Breath-by-breath oxygen uptake (VO2, L/min), carbon dioxide output (VCO2, L/min), tidal volume (VT, L), and minute ventilation (VE, L/min) were recorded during the test (CPX/D; Med Graphics, St. Paul, MN, US). Peak work load and peak VO2 were recorded during the last 20 s of the test. The Metabolic Equivalents of Task (METs) were also calculated. Changes in operational lung volumes were assessed every 2 min during exercise and at peak exercise. Both dyspnea and leg fatigue induced by CPET were measured at the end of the exercise by a 0–100 visual analogue scale (VAS).
Further details on CPET are reported in the supplementary file.
Statistical analysisA Shapiro-Wilk test was used to assess the distribution of the variables. Data were reported as mean ± standard deviation (SD) for the variables with normal distribution and as median [25th – 75th percentile] for those with a non-normal distribution. Unpaired t-test, Mann-Whitney test, and Pearson's Chi square test were used for comparisons when appropriate. Relationships between variables were assessed by Pearson correlation coefficient (r) or by Spearman's rank correlation coefficient (rho), depending on distribution. Linear regression analysis was carried out for values reporting significant correlation. The receiver operating characteristic (ROC) curve was used to plot the true positive rate (sensitivity) in function of the false-positive rate (100-specificity) for a cutoff point of VO2 in mL/kg/min with respect to mMRC ≥ 2 and CAT ≥ 10 as threshold values.19 To test the interrater agreement between CAT and mMRC Cohen's Kappa (κ) was calculated.20 Κ< 0.00 indicates “poor”, 0.00 ≤ κ ≤ 0.02 “slight”, 0.21 ≤ κ ≤ 0.40 “fair”, 0.41 ≤ κ ≤ 0.60 “moderate”, 0.61 ≤ κ ≤ 0.80 “substantial” and 0.81 ≤ κ ≤ 1.00 “perfect” agreement. K was calculated both in all patients and in two subgroups of patients, subdivided according to the median value of the VO2peak.
A p value <0.05 was considered significant.
ResultsWe studied 118 consecutive COPD patients (39 females), aged between 47 and 85 years. Patient's characteristics are shown in Table 1. At study entrance, patients were treated with long-acting beta2-agonists (87%), long-acting muscarinic antagonists (73%) and inhaled steroids (62%). All patients were ex-smokers (64%) or current smokers (36%).
Anthropometric, clinical and lung function characteristics of 118 COPD patients (39 females).
Notes: Values are expressed as mean ± SD or median [25th – 75th percentile] and (range).
In all patients, a wide range of airflow obstruction and lung hyperinflation was found (FEV1/FVC from 26% to 70% and RV/TLC from 30% to 84%, respectively). CAT and mMRC values ranged respectively from 1 to 33 and from 0 to 4 (Table 1) and were positively related (rho = 0.434, p = 0.001). Moreover, CAT and mMRC showed a negative correlation with FEV1/VC (rho = - 0.223, p = 0.02) and (rho = - 0.327, p = 0.001) and a positive correlation with RV/TLC values (rho = 0.208, p = 0.035) and (rho = 0.270, p = 0.01), respectively.
All patients underwent CPET without complications. Mean peak VO2 values in absolute value and as percent of predicted were respectively 15.5 mL/kg/min ± 4.1 and 65% ± 20, while peak workload was 78 W ± 31 (Table 2). Table 3 lists the relationships between CAT scores and mMRC scale scores and exercise variables in all patients.
Exercise characteristics of 118 COPD patients (39 females).
Notes: Values are expressed as mean ± SD and (range).
Relationships between CAT score and mMRC scale score and exercise variables in 118 COPD patients.
According to the ROC curve method, the plot of true-positive rate in function of false-positive rate for a cutoff point of VO2 with respect to a CAT ≥ 10 as threshold value showed 0.703 area under curve value (p = 0.001). A cutoff point, which maximized sensitivity and specificity, was VO2 < 15.7 mL/kg/min (0.64 sensitivity and 0.71 specificity) (Fig. 1). In addition, the plot of true-positive rate in function of false-positive rate for a cutoff point of VO2 with respect to a mMRC ≥ 2 as threshold value showed 0.739 area under curve value (p = 0.001). A cutoff point, which maximized sensitivity and specificity, was VO2 < 15.6 mL/kg/min (0.58 sensitivity and 0.81 specificity) (Fig. 2).
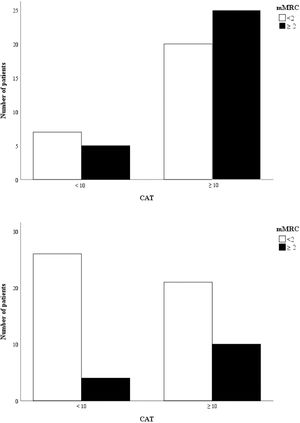
Bars represent the number of patients categorized according to CAT and mMRC and subdivided in two groups, i.e. patients with VO2peak < 15 mL/kg/min (n. 58) (upper panel) and patients with VO2peak ≥ 15 mL/kg/min (n. 62) (lower panel). The interrater agreement between CAT (≥ 10) and mMRC (≥ 2) was slight, κ = 0.10 (upper panel) and κ = 0.20 (lower panel).
In all patients, the interrater agreement between CAT (≥ 10) and mMRC (≥ 2) was found to be fair (κ = 0.21). However, when patients where subdivided according to the median value of the VO2peak (15 mL/kg/min) in those with VO2 peak < 15 mL/kg/min (n. 58) and those with VO2peak ≥ 15 mL/kg/min (n. 62), the interrater agreement between CAT (≥ 10) and mMRC (≥ 2) was slight, κ = 0.10 and κ = 0.20 respectively.
DiscussionThe main finding of the present study is that CAT and mMRC are strictly associated to the maximal exercise capacity in a large cohort of COPD patients with different degrees of severity. Higher scores of CAT or mMRC indicate poorer exercise tolerance. This study also confirmed that CAT and mMRC are related to baseline lung function of the patients, both in terms of airflow obstruction and lung hyperinflation.
The findings of the present study further support the value of CAT and mMRC in the integrated multidimensional management of COPD patients. However, our results show that the agreement between CAT (≥ 10) and mMRC (≥ 2) to categorize COPD patients according to the maximal exercise capacity was slight and, accordingly, this study does not support the use of the recommended cutoff points of ≥ 10 for CAT and ≥ 2 for mMRC as equivalents in the grading of exercise limitation of COPD patients.
The CAT was firstly created and developed to quantify COPD impact in routine practice and to aid patient's health status assessment and communication between patient and physician.21 The CAT was then acknowledged as an accurate and reliable measure of health-related quality of life in COPD patients.22,23 Dyspnea is the most frequent symptom reported by patients suffering from COPD,24 and the mMRC scale is the most commonly used validated scale to assess dyspnea in daily living of these patients.25,26 It is worth noting that the GOLD document recommends the use of both CAT and mMRC to assess symptoms and assign patients to treatment groups.1
Our main findings confirmed and extended the results from previous studies that investigated the relationship between CAT score,13 or mMRC score,14,15 and exercise capacity in COPD patients. In the present study, we provided the evidence that in COPD patients CAT and mMRC scores were inversely related with the maximal oxygen uptake both in terms of mL/kg/min and predicted value and of METs as well as with the maximal workload. Moreover, in our patients we found that a CAT score ≥ 10 and a mMRC score ≥ 2 are very likely to be associated to a value of VO2 peak < 15.7 mL/kg/min and < 15.6 mL/kg/min, respectively. CAT and mMRC were also negatively related to IC at the peak of exercise, which is a measure of dynamic hyperinflation on exertion, and were positively related both to exertional dyspnea and to fatigue. Interestingly, a previous study showed that in COPD patients clinically relevant fatigue was associated with increasing total CAT score and CAT score ⩾10, independently of age, airflow obstruction degree and concomitant heart disease or depression.27 In addition, it has been previously reported that COPD patients who were more dyspneic in their daily living by mMRC scale, were found to complain of more dyspnea after CPET.15
In the present study, CAT score was positively related to mMRC and, interestingly, the cutoff points of CAT (≥ 10) and mMRC (≥ 2) have a high likelihood of being associated to a value of VO2 peak approximately less than 15 mL/kg/min. Interestingly, the cutoff value of 15 mL/kg/min VO2 peak has a potential to discriminate respiratory patients with different grading of functional status. In this study, patients with a VO2 peak value less than 15 mL/kg/min had a significant poorer spirometry than patients with a VO2 peak value greater than 15 mL/kg/min (data not shown). Moreover, it is well known that patients with lung cancer with 15 mL/kg/min VO2 peak, being considered for resection surgery are considered as at increased risk of perioperative complications.28,29
It is worth noting that the interrater agreement between CAT (≥ 10) and mMRC (≥ 2) was found to be fair in all patients and even slight, when patients where subdivided into two subgroups, i.e. in patients with VO2peak < 15 mL/kg/min and in patients with VO2peak ≥ 15 mL/kg/min. Our finding is the first evidence about the agreement between CAT and mMRC in relation to maximal exercise capacity. A previous systematic review and meta-analysis specifically addressed the evaluation of the agreement between patient's assignment into GOLD categories using CAT cut point ≥ 10) or mMRC cut point ≥ 2 showed a misclassification of 13% in all GOLD categories, agreement ranging between CAT and mMRC from poor to substantial (κ value 0.13 to 0.77).23
This study has some strengths: the large size of the patient's sample and the prospective and consecutive nature of the data collection. On the other hand, we acknowledge that this study also has some limitations. Firstly, our study is a cross-sectional study and our results may be of value in the assessment of the functional status of patients with COPD, but not in their prognostic evaluation. Thus, a further longitudinal study on exercise tolerance in COPD patients, who change their health-related quality of life over time, is needed. Secondly, in our study patients experienced maximal exercise capacity by using cycle ergometry, therefore, our results cannot be applicable to other forms of exercise, such as running on a treadmill, where the metabolic load is greater.
ConclusionsIn conclusion, we demonstrated that CAT as well as mMRC are useful tools to predict exercise tolerance in COPD. Furthermore, in our patients CAT and mMRC were significantly related to dynamic hyperinflation on exertion and to exertional dyspnea and fatigue. However, we found that the agreement between the cutoff points of CAT (≥ 10) and mMRC (≥ 2) was poor, when related to maximal exercise capacity and, accordingly, they cannot be considered as supplementary measures.
FundingThis work was not supported by any financial source.
None.