Chronic Obstructive Pulmonary Disease (COPD) is a heterogeneous and multisystemic disease with progressive increasing morbidity and mortality. COPD is now widely accepted as a heterogeneous condition with multiple phenotypes and endotypes. This review will discuss the old and new concepts for the different types of COPD phenotypes, as well as the inclusion of them in current guidelines. Phenotypical approach to COPD is having huge impact on everyday practice and changed nonpharmacological and pharmacological management of COPD in last decade. However, phenotypical approach is small step to precision medicine in COPD management in the absence of big, specific and well-designed COPD trials with exact identification of phenotypes for more personalization of the treatment of COPD.
The most recent update of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) defined the concept of Chronic Obstructive Pulmonary Disease (COPD) as a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and or alveolar abnormalities, usually produced by major exposure to harmful particles or fumes.1 It is a leading cause of morbimortality worldwide that is substantially increasing.2
In more recent years, the term phenotype has been introduced to help clinicians in the identification of the different types of COPD subgroups. The definition for “phenotype” is considered as “the physical appearance or biochemical characteristic as a result of an interaction between the genotype and environment”. Moreover, the definition clearly states that a phenotype has to be a subgroup with a great impact in the prognosis (symptoms, exacerbations, response to therapy, rate of disease progression, or death).3
The first to have the idea of conceptualizing the different types of phenotypes was Snider, in 1989. With a nonproportional Venn diagram, the classic 3 subgroups of COPD were introduced: chronic bronchitis, emphysema, and asthma in three overlapping circles. Those subsets of patients were known to continuously have airway obstruction.4 That concept was included in the 1995 American Thoracic Society (ATS) COPD guidelines.5
In the years after that concept was proposed, several studies have shown that the overlap is undoubtedly significant, and the knowledge of the pathogenesis has evolved over time, as well as the clinical characteristics. This overlap can be challenging for some clinicians because of the imprecision of the concepts and the different recommendations for the management from current respiratory guidelines.6 Thus, the heterogeneity of these conditions led to the importance of phenotyping even though the patients share some features of two or even three of these conditions. Some clinicians may benefit from this classification to predict the outcome and set a specific patient-therapy.7
But from these phenotypes, the one that is likely to be considered as an entity on its own, or even as a separate syndrome is asthma. Some hypotheses divide asthma into more specific syndromes with distinct “endotypes”. An endotype is proposed to be a subtype of a condition defined by a different pathophysiological mechanism.8 This was proposed after some characteristics of asthma were not always present in large cohorts of patients, such as recurring symptoms, airflow obstruction, bronchial hyperreactivity and underlying inflammatory response.9 Lötvall et al., proposed rules for defining the asthma endotypes after selecting 7 parameters such as clinical characteristics, biomarkers, lung physiology, genetics, histopathology, epidemiology, and treatment response. That resulted in 6 different phenotypes and 5 separate endotypes and the ideal approach for these patients should be endotype-specific.10
Another classic phenotype is emphysema, which is a significant component of COPD and the extent increases with increasing the severity of airflow limitation, making that subgroup a very stable phenotype. The same concept can be stated with chronic bronchitis, which is been associated with excess forced expiratory volume in the first second (FEV1) and is observed predominantly in young adults.11 One clinical feature of the different types of phenotypes is the frequency and severity of exacerbations. Several clinical trials have demonstrated that only a small number of patients with COPD experienced exacerbations. The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study which included 2138 patients established that a patient who suffered 2 or more exacerbations per year was classified with a stable phenotype. The exacerbations were defined as events that led a care provider to prescribe antibiotics or corticosteroids, as well as hospitalization. The exacerbation frequency was followed over a period of 3 years.12 This has a great impact in the COPD prognosis because exacerbations are linked to a bad prognosis and an excess FEV1 decline.
The purpose of this article is to review the old and new concepts for the different types of COPD phenotypes, as well as the inclusion of them in current guidelines (Table 1).
Classification of COPD phenotypes.
| Widely accepted COPD phenotypes | Emerging COPD phenotypes |
|---|---|
| Chronic BronchiticEmphysematousAsthma-COPD-OverlapFrequent exacerbatorRare exacerbator | Pulmonary cachexia phenotypeOverlap COPD and bronchiectasisUpper lobe-predominant Emphysema PhenotypeThe fast decliner phenotypeThe comorbidities or systemic phenotypeα1-Antitrypsin DeficiencyNo smoking COPD |
Old classifications of phenotypes were A (Patients with chronic bronchitis, inflammatory phenotype, frequent exacerbator, systemic manifestations and with co-morbidities) and B (Patients with emphysema, pronounced lung hyperinflation and without frequent exacerbations). But the reality is that many more phenotypes are likely to exist, and all of them have become almost synonymous with a clinical subgroup, leading to limited alternatives for the pharmacological treatment. It is important to mention that although a reduction of the ratio of FEV1 to forced vital capacity (FVC) has been adopted as an unquestionable sign of airflow obstruction, there is no consensus of cut-off achievement to separate healthy patients from obstructive patients. This will make any definition of phenotype that has been described after a prospective analysis to be somehow inconsistent, especially when a based fixed FEV1/FVC<0.70 ratio was not used. This fixed ratio has been included in GOLD and also by the British National Institute for Health and Clinical Excellence (NICE) and the Canadian Thoracic Society. But this does not imply that the diagnosis with COPD using a fixed ratio is more accurate and confirmed than using the FEV1/FVC below the lower 5th percentile or lower limit of normality (LLN), which decreases with age.13 Because this definition leaves a large proportion of subjects with physiological abnormalities that also manifest respiratory symptoms but do not satisfy the COPD diagnostic criteria, the patients will need to be approached from multiple dimensions (clinical, physiological, imaging and endotyping).14
Vestbo et al. suggested in 2014 the following phenotypes: asthma, bronchial hyperresponsiveness, bronchodilator reversibility, emphysema, hyperinflation, cachexia, chronic bronchitis, frequent exacerbations, and systemic inflammation.15 However, this classification has changed over the years since the original idea. Weatherall et al., used a cluster analysis to explore the clinical phenotypes in a community population with airways disease. That analysis included 175 subjects and 5 clinical phenotypes were identified: 1) severe and markedly variable airflow obstruction with features of atopic asthma, chronic bronchitis, and emphysema; 2) features of emphysema alone; 3) atopic asthma with eosinophilic airways inflammation, 4) mild airflow obstruction without other dominant phenotypic features and 5) chronic bronchitis in nonsmokers.16 The concept of clinical phenotype in COPD emerged as those attributes of the disease alone or in combination that describe differences between individuals with COPD in relation to parameters that have significance (i.e. symptoms, exacerbations, treatment response, progression of the disease or death).3 These findings are relevant in establishing the phenotype response to various pharmacological treatments and to providing the most appropriate treatment.17 For example, phosphodiesterase-4 inhibitors (Roflumilast or cilomilast) are only used in patients with chronic bronchitis and they help them in improving the likelihood of exacerbations. On the other hand, patients with COPD-asthma overlap phenotype show an enhanced response to inhaled corticosteroids.18,19
The use of analytic approaches, like cluster analysis, has advanced the study of phenotypes. They facilitate the identification of unique groups of related variables in an attempt to recognize features that might relate to both underlying disease biologically and clinically significant outcomes. Those approaches usually resulted from the use of data obtained from large cohorts of well-characterized patients to identify their relation between clinical variables and outcomes.20 One of those analyses was the ECLIPSE12 which provided significant information about the susceptibility of certain patients to develop exacerbations. An additional study by the Phenotype and Course of COPD subclassified groups of COPD patients with exacerbations. In this last mentioned study, 342 subjects with COPD who were hospitalized with their first exacerbation, were identified as belonging to 3 distinct COPD groups: 1) “severe respiratory COPD” characterized by airflow limitation (mean FEV1, 38% of predicted value), 2) “moderate respiratory COPD” marked by milder degrees of airflow limitation (mean FEV1, 58% of predicted value), 3) “systemic COPD” with similar milder airflow limitation but with a greater proportion of comorbidities such as obesity, cardiovascular disease and diabetes mellitus.21 But there are many other ways of classifying phenotypes, like radiologic data that could be associated with some clinical features of COPD phenotypes. The COPDGene study was a multicenter observational study designed to identify genetic factors with COPD. Subjects of that study underwent inspiratory, whole-lung volumetric multidetector computed tomography (CT)22 with measurement of total lung emphysema percentage. Using the data of that trial, Han and collaborators were able to determine that both bronchial wall thickness and total lung emphysema percentage were predictive factor of COPD exacerbation frequency in a continuous way independently of severity airflow limitation.23
But then again these phenotypes always have a physiologic explanation, and the classic Fletcher-Peto curve published in 1976, has been used over time to define susceptibility phenotypes of patients with COPD based on the rate of lung function decrease; the relationship between the variables (FEV1, age, smoking history) is still not well understood. This is because they stated that as airflow worsens, the symptoms increase but this varies enormously among individual patients.24 Some researchers have been studying these variables and their relationship using prospective studies. Nishimura et al did a 5-year prospective follow-up study of patients with COPD in Japan to identify variables that might influence the rate of COPD progression. They evaluated lung function and CT scan results at baseline and twice-yearly lung function and clinical outcomes over the follow-up period. It resulted in a cohort with 3 groups: 1) sustained lung function, 2) slow rate of lung function decrease [30mL/year decrease in FEV1] and 3) rapid rate of lung function decrease [60ml/year decrease in FEV1].25 The patients with sustained lung function had less evidence of emphysema and a higher number of circulating eosinophils, in comparison with those rapid progressors who had the highest ratio of emphysema demonstrated on CT scans and a lowest transfer coefficient for carbon monoxide.
At the same time, circulating eosinophils will need to be evaluated to differentiate airway eosinophilia and airway hyperresponsiveness. A prospective clinical study made by Kume et al examined the prevalence of airway eosinophilia and airway responsiveness in COPD patients who have neither symptoms nor past medical history of asthma and also explored the association of these pathophysiological features of asthma in the management for COPD. This was done by sputum qualitative and quantitative analysis in patients with COPD GOLD stage 1-3. Sputum eosinophils were observed in 65 subjects of 129 (50.4%) using qualitative analysis. Airway hyperresponsiveness was developed in 46.9% of these subjects, and the exacerbations were more frequently in lower-grade airway eosinophilia without ciclesonide than higher-grade airway eosinophilia with ciclesonide. These entities are characteristic features of asthma, but they may develop in the subject of patients with COPD who do not present any symptoms related to asthma or a previous diagnosis of asthma.26
Another characteristic of asthma is wheezing, and not all the COPD patients presented with wheezing and this could be another clinical phenotype that would help differentiate subgroups. The Taiwan Obstructive Lung Disease study was a retrospective, multicenter research study which assessed medical records from patients with COPD over 40 years, between November 2012 and August 2013. Here patients with asthma were excluded, and demographic data, lung function, symptom scores and frequency of acute exacerbations were recorded and analyzed. Also, they evaluated the differences between patients with and without wheezing. From 1096 patients with COPD, only 424 (38.7%) had wheezing phenotype, and from this group they had more acute exacerbations within the past year analyzed than the non-wheezing group. The postbronchodilator FEV1 was lower in wheezing patients (p<0.001) associating these patients with a worse COPD phenotype in comparison to those without that symptom.27
More important when trying to classify a patient into a phenotype, is to consider the tobacco smoke exposure history and the use of cigarettes. Even though smoking is a major risk factor for COPD, more than ¼ of COPD patients are non-smokers. A Korean cohort study made by Ji et al.28 near a cement plant, observed smokers and non-smokers by a cutoff of a 5 pack-year smoking history. The non-smoker (n=49) group resulted in younger patients with a superior BMI vs. the smoker group (n=11) (p<0.05). The smokers group had more emphysema than non-smokers but with a borderline statistical significance (p=0.051). In this study the tobacco smoke exposure was highly associated with an emphysema phenotype, while exposure to biomass (i.e. cement) exhibited less emphysema and more air trapping and more structural lung changes on volumetric CT scans (Table 2).
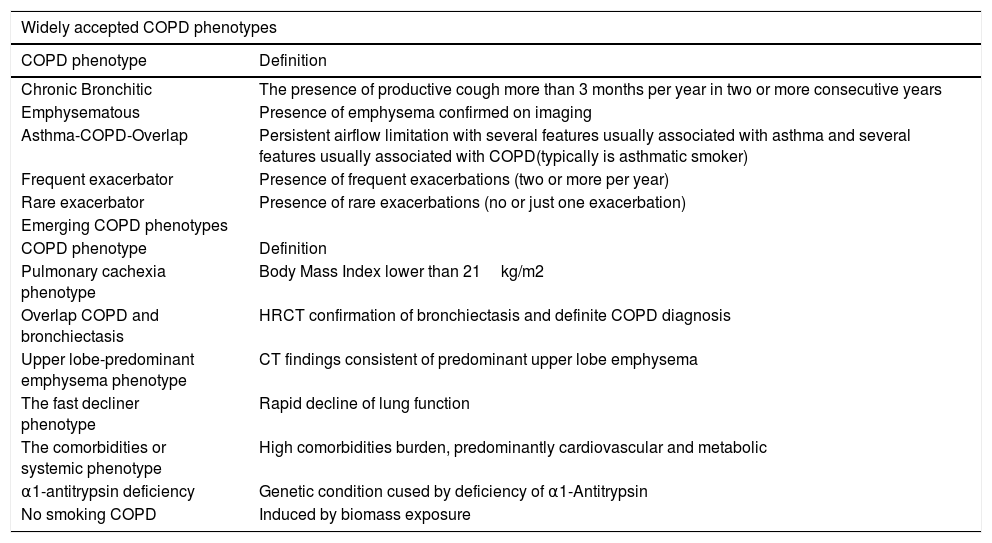
Definitions of widely accepted and emerging COPD phenotypes.
| Widely accepted COPD phenotypes | |
|---|---|
| COPD phenotype | Definition |
| Chronic Bronchitic | The presence of productive cough more than 3 months per year in two or more consecutive years |
| Emphysematous | Presence of emphysema confirmed on imaging |
| Asthma-COPD-Overlap | Persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD(typically is asthmatic smoker) |
| Frequent exacerbator | Presence of frequent exacerbations (two or more per year) |
| Rare exacerbator | Presence of rare exacerbations (no or just one exacerbation) |
| Emerging COPD phenotypes | |
| COPD phenotype | Definition |
| Pulmonary cachexia phenotype | Body Mass Index lower than 21kg/m2 |
| Overlap COPD and bronchiectasis | HRCT confirmation of bronchiectasis and definite COPD diagnosis |
| Upper lobe-predominant emphysema phenotype | CT findings consistent of predominant upper lobe emphysema |
| The fast decliner phenotype | Rapid decline of lung function |
| The comorbidities or systemic phenotype | High comorbidities burden, predominantly cardiovascular and metabolic |
| α1-antitrypsin deficiency | Genetic condition cused by deficiency of α1-Antitrypsin |
| No smoking COPD | Induced by biomass exposure |
A similar retrospective study was done in Spain by Golpe et al.,29 where he observed 499 patients diagnosed with COPD by smoking or biomass exposure. Here 122 were classified into biomass exposure subgroup and 377 to the tobacco exposure. Male gender was higher in the tobacco group (92.1%) and there was more frequency of emphysema among the tobacco users. Prevalence of chronic bronchitis and exacerbations, comorbidities and hospital admission rate were equal between both groups. Similar results were found in a cross-sectional study where women exposed to biomass smoke, never-smokers and former smokers were observed in a COPD clinic in Mexico City, Mexico. In this study, women in the tobacco group had more significantly marked emphysema than in the biomass group. And in the biomass group these women had more air trapping than the tobacco group.30 This study complemented the existing information about the significant differences in the clinical presentation of this phenotype, the pulmonary function test and CT finding between biomass group (such as wood, charcoal, grass or crop smoke) and tobacco smoke-related COPD. A revision by Torres-Duque et al.31,32 reinforces the fact that wood smoke is a completely different phenotype.
The World Health Organization (WHO) estimates that biomass smoke exposure, or household air pollution, is responsible for 4.3 million deaths annually globally, with particular attention to South East Asian and Western Pacific regions. And even though biomass exposure and tobacco exposure are both associated with similar reductions in post-bronchodilator airflow obstruction, biomass exposure shows greater reduction in mid-expiratory flow and less pronounced markers of emphysema like air trapping. Moreover, it is documented that in biomass smoke exposure there is thickening of the basement membrane and lymphocytic predominance in a bronchoalveolar lavage fluid and some visualization of bronchial anthracofibrosis.33
However, in the above-mentioned study, there was a mixed COPD-asthma phenotype which was more prevalent in the biomass exposure subgroup.31 And in more recent years, it has increased attention to the developement of this new COPD-asthma syndrome/phenotype that combined can affect a total of more than 60 million patients globally34 and which is why the NOVELTY35 study was originated in 2016. This trial is planned to end in 2021, and it is a global, prospective observational 3-year study enrolling ˜12000 patients over 12 years of age from primary and specialist clinical practices in 19 countries (ClinicalTrials.gov identifier: NCT02760329). The primary objective of this study is to describe patient characteristics, treatment designs and disease burden over time, as well as to identify phenotypes and molecular endotypes that are associated with differential aftermaths throughout the time patients have suspected or even diagnosed asthma and COPD. The countries participating in this project are Argentina, Australia, Brazil, Canada, China, Colombia, Denmark, France, Germany, Italy, Japan, Mexico, the Netherlands, Norway, South Korea, Spain, Sweden, UK and USA. The results of this study will enable accurate patient classification according to the clinical outcomes and the biomarker profiles, and consequentially support the development of advanced personalized therapies.
In spite of this, these studies were often performed in unselected populations of COPD and it is possible that different results could have been detected in selected subpopulations. As a consequence, there is a real need for larger studies to identify variables other than lung function to improve the risk assessment in patients with COPD.18,36 This will benefit the clinicians for a development of COPD management guidelines.
Phenotyping and current COPD guidelinesThe first Spanish COPD guidelines (GesEPOC) were developed in 2012 and it was one of the very early attempts to introduce the phenotypical approach into clinical practice.37
In 2016 a detailed analysis of national COPD guidelines across Europe and Russia was published.38 This demonstrated high variability across national guidelines according to the detection of COPD phenotypes, determined probably by the different time of publishing of COPD guidelines. The classic COPD phenotypes of chronic bronchitis and emphysema were recognised in guidelines from the Czech Republic, England and Wales, Poland, Russia, Spain and Sweden (bronchitic only).
In the 2019 GOLD guidelines management of stable COPD was redefined: groups A, B, C, D are now used just for informing the initial treatment only.1 Regarding follow-up, two outcomes are proposed: dyspnoea and exacerbations, with different individualized treatment algorithms. Blood eosinophil count is introduced as a biomarker for the likelihood to treatment with an inhaled corticosteroids.
The GOLD guidelines help the clinicians in the diagnosis and treatment of COPD. However, COPD is a very complex disease with a high index of morbimortality. Efforts to identify subgroups or phenotypes have been a challenge that has evolved with time. Siafakas et al recommend some modes of treatments by phenotyping the patient before starting therapy, but also noticed the lack of strong cluster studies.39 As the medicine and technology advance, ongoing research highlights such biomarkers, have been incorporated into clinical guidelines, as the basis of clinical phenotypes.
Impact of phenotyping in the management of COPDPhenotypical approach to COPD is having huge impact on everyday practice and haschanged nonpharmacological and pharmacological management of COPD in last decade.
In recent years some common reasons for the multiple failures of drug development in COPD have been described and analyzed, including inadequate target engagement of the drug, poor patient selection and use of clinical endpoints that are insensitive or inaccurate for detecting appropriate treatment responses.40,41 It was shown that use of biomarkers for COPD phenotype patients and selecting only those who are likely to experience benefit from the drug dramatically increases the probability of success of novel drugs in phase IIa trials from ˜29% (pre-biomarker implementation) to 82% (with biomarker implementation).40,41 Probably this combined complex approach with application of COPD phenotypes and new biomarkers will revolutionize COPD management in next years.
ConclusionsPhenotypical approach to COPD is having huge impact on everyday practice and has changed nonpharmacological and pharmacological management of COPD in last decade. However, phenotypical approach is small step towards precision medicine in COPD management in the absence of big, specific and well-designed COPD trials with exact identification of phenotypes for more personalization of the treatment of COPD.
Author contributionsAll authors equally contributed to this paper with conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of the final version.
Conflict-of-interest statementNo potential conflicts of interest. No financial support.
None.