University-affiliated hospital located in Porto, North Portugal, an area with a low to intermediate incidence of tuberculosis (TB).
ObjectiveTo identify predictors and outcomes of disseminated TB (dTB).
DesignA cohort of patients diagnosed with TB between 2007 and 2013 was retrospectively analysed. Patients with dTB criteria were characterized and compared to single organ TB cases. Factors independently associated with dTB were determined by multivariate logistic regression analysis.
ResultsA total of 744 patients were analysed, including 145 with dTB. Independent risk factors for dTB were pharmacological immunosuppression (OR 5.6, 95% CI 2.8–11.3), HIV infection (OR 5.1, 95% CI 3.1–8.3), chronic liver failure or cirrhosis (OR 2.3, 95% CI 1.4–4.1) and duration of symptoms (OR 2.3, 95% CI 1.4–3.8). Compared to single organ TB, the clinical presentation of dTB patients differed by the absence of haemoptysis (OR 3.2, 95% CI 1.3–8.4) and of dyspnoea (OR 1.9, 95% CI 1.2–3.1), presence of weight loss (OR 1.8, 95% CI 1.1–2.9), night sweats (OR 1.7, 95% CI 1.1–2.7) and bilateral lung involvement (OR 4.4, 95% CI 2.8–7.1). Mortality and time until culture conversion were higher for dTB patients, although not reaching statistical significance.
ConclusionImmunosuppressive conditions and chronic liver failure or cirrhosis were associated with increased risk of dTB. The haematogenous spread may be dependent on longer symptomatic disease and usually progresses with bilateral lung involvement.
While lungs are the organs primarily affected in TB, accounting for nearly 70% of TB cases, infection can also affect other organs through lymphohematogenous spread.1,2 Typical extrapulmonary disease locations include lymph nodes, pleura, genitourinary tract, bones and joints, meninges, peritoneum and pericardium.2,3 Disseminated TB (dTB) has been increasingly observed in immunocompromised hosts,4,5 especially in developing countries, where dTB is a major cause of morbidity and mortality due to higher rates of TB-human immunodeficiency virus (HIV) co-infection.1 It is often difficult to establish diagnosis, and the clinical presentation of dTB is commonly non-specific, with symptoms varying according to the affected organs.2,4,5 Retinal choroidal tubercles are a pathognomonic finding of dTB, they are identified in only about 5–20% of the cases.6 Similarly, the classic chest X-ray features of miliary TB may help in the diagnosis, although less than a third of dTB patients may present them.7 One study revealed that 33–80% of dTB cases were only diagnosed post-mortem, during autopsies.8 Thus, diagnostic delay is probably one of the main reasons why dTB is associated with higher mortality rates than pulmonary TB (pTB).1,2,4
Therefore, it is essential to recognize the most important risk factors for dTB, because a strong clinical suspicion may be the key towards early diagnosis and consequent beginning of effective treatment. Our research aims to contribute to the study of clinical predictors associated with dTB, which may be helpful for more effective prevention and intervention strategies.
MethodsStudy design and patient populationThe present work was based in an intermediate burden setting, in the second largest urban area in Portugal, Porto, where the incidence rate reached 46.3/100,000 inhabitants in 2014.9 We conducted a retrospective analysis of the clinical records of patients with culture-confirmed TB at a University-affiliated hospital (Centro Hospitalar São João – CHSJ) during a 7-year period (2007–2013).10 TB cases were defined according to the World Health Organization guidelines and treatment was administered by directly observed treatment 5–7 days/week, with the recommended treatment regimens.11 Patients aged <18 years or whose clinical information was lacking (no registries found) were excluded.
Subjects were categorized according to the disease site: dTB or locoregional TB. Disseminated TB was defined by any of the following situations: (i) isolation of Mycobacterium tuberculosis in the blood stream (positive blood cultures), bone marrow, liver biopsy samples, or in ≥2 non-contiguous organs; (ii) isolation of M. tuberculosis in one organ and histological confirmation of caseating granulomas in the bone marrow, liver biopsy samples, or in another non-contiguous site; (iii) isolation of M. tuberculosis in any organ with miliary pattern on the chest X-ray.4 Patients with isolated hepatic abscess, or with pTB along with cervical or axillar lymph nodes involvement were considered to have locoregional disease.4,12 This study conforms to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.13
Data collectionData were collected from both the CHSJ clinical files and the Portuguese regional surveillance system (SVIG-TB) database. Sociodemographic information, lifestyle factors and the presence of comorbidities were registered as defined in a previous report.10 Baseline clinical features available at time of diagnosis were also collected. These included: time of symptoms onset; the presence of respiratory and constitutional symptoms; haemoglobin and C-reactive protein (CRP) values; and acute hypoxaemic respiratory failure.10 Baseline chest radiographs were analyzed blindly by two independent physicians according to the pulmonary extension of the disease, presence of cavitation and/or pleural effusion and their dimensions (> or <4cm, and >or <2cm from carina, respectively). Disagreement between readers was resolved through a consensus which was read by a third physician. Most cases had microscopic examination of auramine-stained sputum slides, ranking acid-fast bacilli load as negative, 1+, 2+ or 3+,14 and a drug susceptibility profile was also available.
Time until sputum culture conversion (difference between the 1st day of treatment and the 1st negative culture) was also registered. Deaths that occurred during the first 6 months after diagnosis were classified as TB related.10,11
Statistical analysisUnivariate analyses were conducted to compare features of dTB and locoregional TB cases. Differences between groups were assessed using independent group t-test, or Mann–Whitney U-test for non-normally distributed variables. For categorical variables Chi-square test or Fisher exact test were used as appropriate. Multivariable logistic regression was used to determine independent predictors for dTB. Due to lack of information in some variables (Table 1), multiple imputation was applied.15 All variables associated with dTB with a p<0.20 in univariate analysis were introduced in the multivariate model. To identify the independent and significant factors associated with dTB, the backward method of variable selection was applied. On the construction of the model, the variables that were not significant or where collinearity was present were excluded. Variables were considered statistically significant when p<0.05. The results of significant predictors were reported as odds ratios (OR) and with confidence interval (CI) of 95%. The accuracy of the model was evaluated using the receiving operator characteristic (ROC) analysis and the Hosmer–Lemeshow test. Statistical analysis was performed using the SPSS software programme, version 22 (IBM®/ce:italic>SPSS®/ce:italic>, Inc.).
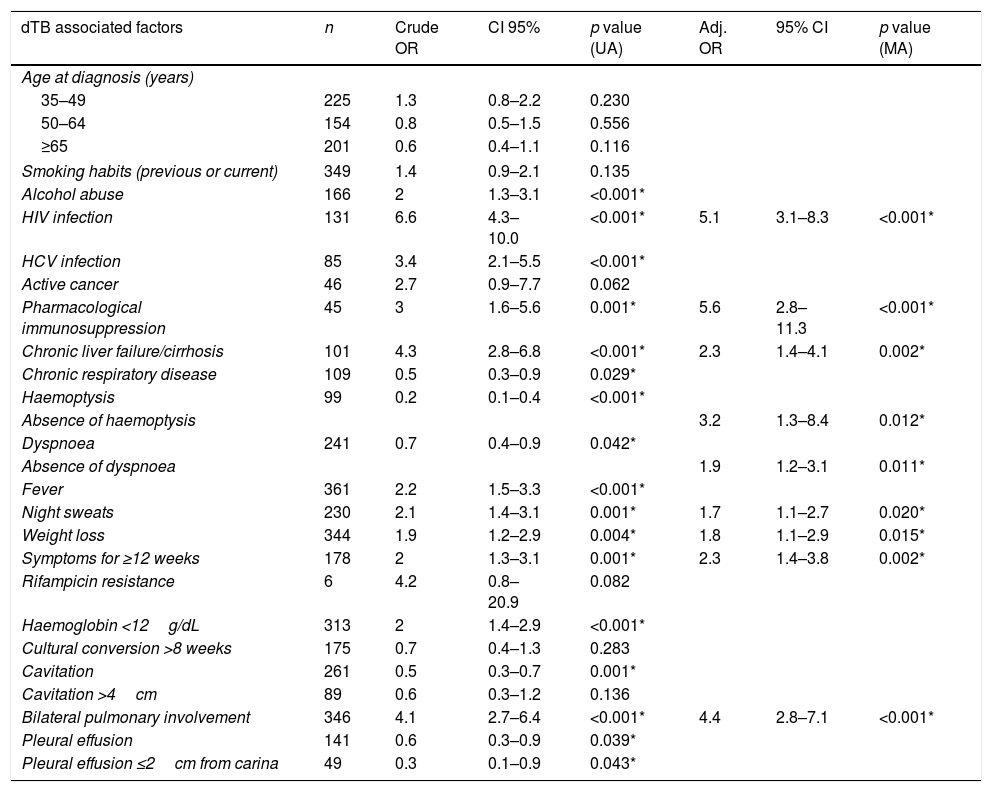
General characteristics of the study cohort and specific associations with dTB. The p values reported derive from univariate analysis.
| General characteristics | No information | Total (n=744) | dTB (n=145) | Locoregional TB (n=599) | p value |
|---|---|---|---|---|---|
| Age, mean±SD | 0 | 51.0±18.9 | 48.4±17.2 | 51.6±19.2 | 0.051 |
| Male gender, n (%) | 0 | 535 (71.9) | 428 (71.5) | 107 (73.8) | 0.646 |
| Previous history of TB, n (%) | 43 (5.8) | 76 (10.8) | 16 (11.3) | 60 (10.7) | 0.948 |
| Smoking habits, n (%) | 150 (20.2) | 0.050 | |||
| Non smoker | 245 (41.2) | 38 (34.9) | 207 (42.7) | ||
| Former smoker | 100 (16.8) | 14 (12.8) | 86 (17.7) | ||
| Smoker | 249 (41.9) | 57 (52.3) | 192 (39.6) | ||
| Alcohol abuse, n (%) | 100 (13.4) | 166 (25.8) | 46 (37.7) | 120 (23.0) | 0.001 |
| HIV infection, n (%) | 76 (10.2) | 131 (19.6) | 66 (47.8) | 65 (12.3) | <0.001 |
| HCV infection, n (%) | 276 (37.1) | 85 (18.2) | 39 (33.6) | 46 (13.1) | <0.001 |
| Active cancer, n (%) | 9 (1.2) | 46 (6.3) | 4 (2.8) | 42 (7.1) | 0.080 |
| Pharmacological immunosuppression n (%) | 6 (0.8) | 45 (6.1) | 18 (12.4) | 27 (4.6) | 0.001 |
| Chronic liver failure/cirrhosis, n (%) | 12 (1.6) | 101 (13.8) | 45 (31.3) | 56 (9.5) | <0.001 |
| Diabetes mellitus, n (%) | 5 (0.7) | 91 (12.3) | 16 (11.0) | 75 (12.6) | 0.702 |
| CKD stage 4–5, n (%) | 6 (0.8) | 46 (6.2) | 8 (5.5) | 38 (6.4) | 0.837 |
| Congestive heart failure, n (%) | 14 (1.9) | 61 (8.4) | 9 (6.3) | 52 (8.9) | 0.395 |
| Chronic respiratory disease, n (%) | 19 (2.6) | 109 (15.0) | 13 (9.1) | 96 (16.5) | 0.037 |
| Cough, n (%) | 149 (20.0) | 445 (74.8) | 90 (70.3) | 355 (76.0) | 0.229 |
| Haemoptysis, n (%) | 154 (20.7) | 99 (16.8) | 6 (4.6) | 93 (20.2) | <0.001a |
| Dyspnoea, n (%) | 147 (19.8) | 241 (40.4) | 42 (32.6) | 199 (42.5) | 0.052 |
| Fever, n (%) | 114 (15.3) | 361 (57.3) | 98 (71.5) | 263 (53.3) | <0.001a |
| Night sweats, n (%) | 206 (27.7) | 230 (42.8) | 66 (56.9) | 164 (38.9) | 0.001a |
| Weight loss, n (%) | 158 (21.2) | 344 (58.7) | 89 (70.1) | 255 (55.6) | 0.005a |
| Hypoxaemic respiratory failure, n (%) | 119 (16.0) | 122 (19.5) | 25 (18.0) | 97 (20.0) | 0.692 |
| Time of symptoms, median weeks (IQR) | 208 (28.0) | 7.0 (4.0–12.0) | 9.0 (4.0–16.0) | 7.0 (3.5–12.0) | 0.032a |
| Bacillary load, n (%) | 262 (35.2) | 0.062 | |||
| 0 | 141 (29.3) | 24 (22.9) | 117 (31.0) | ||
| 1+ | 66 (13.7) | 22 (21.0) | 44 (11.7) | ||
| 2+ | 99 (20.5) | 23 (21.9) | 76 (20.2) | ||
| 3+ | 176 (36.5) | 36 (34.3) | 140 (37.1) | ||
| Anti-TB drugs susceptibility test, n (%) | |||||
| Isoniazid resistant | 30 (4.0) | 35 (4.9) | 7 (5.0) | 28 (4.9) | 0.952 |
| Rifampicin resistant | 30 (4.0) | 6 (0.8) | 3 (2.1) | 3 (0.5) | 0.060 |
| Pyrazinamide resistantb | 421 (56.6) | 7 (2.2) | 1 (2.0) | 6 (2.2) | 0.912 |
| Ethambutol resistant | 30 (4.0) | 7 (1.0) | 1 (0.7) | 6 (1.0) | 0.722 |
| Streptomycin resistant | 31 (4.2) | 86 (12.1) | 16 (11.5) | 70 (12.2) | 0.939 |
| Multidrug resistant, n (%) | 30 (4.0) | 3 (0.4) | 1 (0.7) | 2 (0.3) | 0.481 |
| Haemoglobin (g/dL), mean±SD | 79 (10.6) | 12.0±2.2 | 11.4±2.2 | 12.1±2.2 | <0.001a |
| C reactive protein (mg/L), mean±SD | 104 (14.0) | 84.8±67.6 | 88.7±63.2 | 83.8±68.7 | 0.447 |
| Cavitation, n (%) | 60 (8.1) | 261 (41.4) | 36 (25.5) | 225 (38.2) | 0.001a |
| Cavitation >4cm, n (%) | 60 (8.1) | 89 (13.0) | 13 (9.2) | 76 (14.0) | 0.173 |
| Pulmonary involvement, n (%) | 74 (9.9) | <0.001a | |||
| Not detected | 159 (23.7) | 16 (11.4) | 143 (27.0) | ||
| Unilateral | 165 (24.6) | 16 (11.4) | 149 (28.1) | ||
| Bilateral | 346 (51.6) | 108 (77.7) | 238 (44.9) | ||
| Pleural effusion, n (%) | 10 (1.3) | 141 (19.2) | 19 (13.1) | 122 (20.7) | 0.037a |
| Pleural effusion ≤2cm carina, n (%) | 11 (1.5) | 49 (6.7) | 4 (2.8) | 45 (7.7) | 0.035a |
Definition of abbreviations: CKD: chronic kidney disease; dTB: disseminated tuberculosis; HCV: hepatitis C virus; HIV: human immunodeficiency virus; IQR: interquartile range; TB: tuberculosis.
This study protocol was approved by the Ethics Committee of the CHSJ (approval number 109-11), by the North Health Region Administration (approval number 71-2014) and by the Portuguese Data Protection Authority (approval number (12174-2011). The requirement to obtain informed written consent from each individual was waived, as the study was limited to the review of existing medical records. To ensure confidentiality, each case was anonymized by the assignment of a random identification number.
ResultsSample characterizationBetween 2007 and 2013, 813 culture-confirmed new TB cases were diagnosed at the CHSJ (both inpatient and outpatient).10 Patients <18 years old (n=9), with unavailable data (n=40) or with ambiguous dTB classification (n=20) were excluded. A total of 744 TB cases were analyzed, of which 145 (19.5%) had dTB. The general characteristics of the study cohort are presented in Table 1. The vast majority of cases included in the study were Portuguese-born Caucasians (n=718, 96.5%).
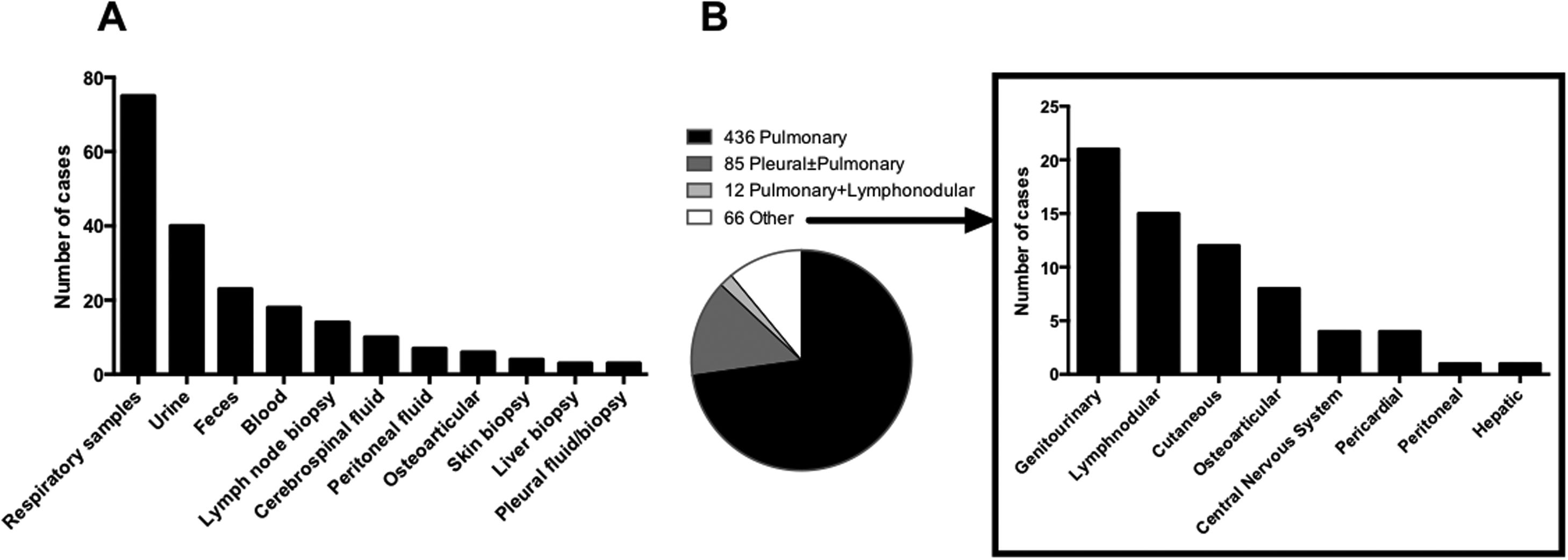
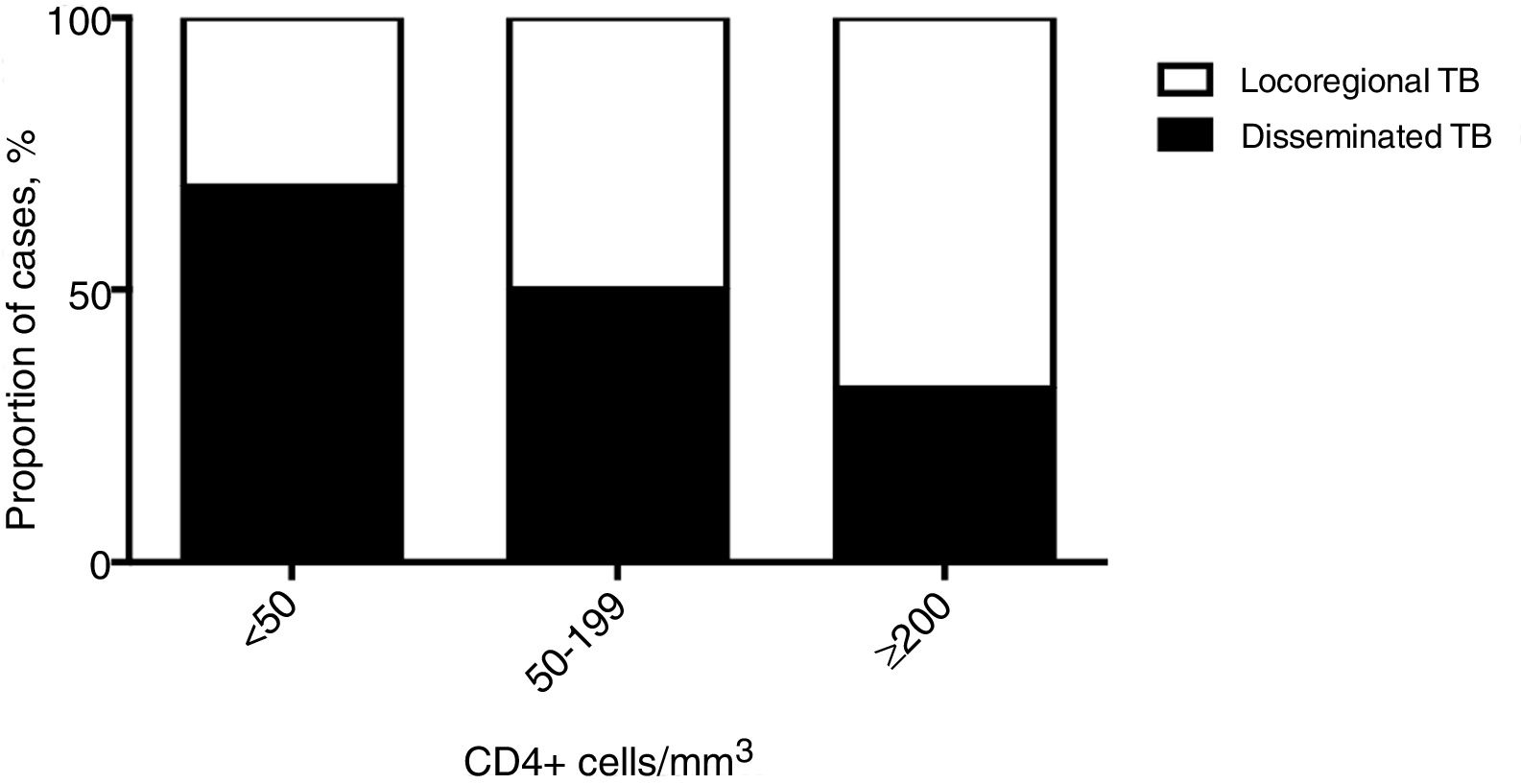
Most patients with dTB had M. tuberculosis isolated in two or more non-contiguous locations (n=103, 71.1%), and 58 (40.0%) cases presented miliary TB. Almost all dTB patients (n=132, 91%) had pulmonary involvement, 32.4% (n=47) had genitourinary and 21.4% (n=31) abdominal involvement, namely intestinal (n=22), peritoneal (n=6), hepatic (n=2), and in one patient all three afore mentioned organs were affected. In 26 (17.9%) cases there was no disease focus apparent, and the diagnosis was established through blood, bone marrow and liver biopsy samples. Fig. 1A summarizes specimens with positive mycobacterial culture of dTB patients. In patients with locoregional TB, pulmonary disease was the most frequent (n=436, 72.3%), followed by pleural±pulmonary (n=85, 14.2%). The remaining types of disease in patients with locoregional TB are represented in Fig. 1B.
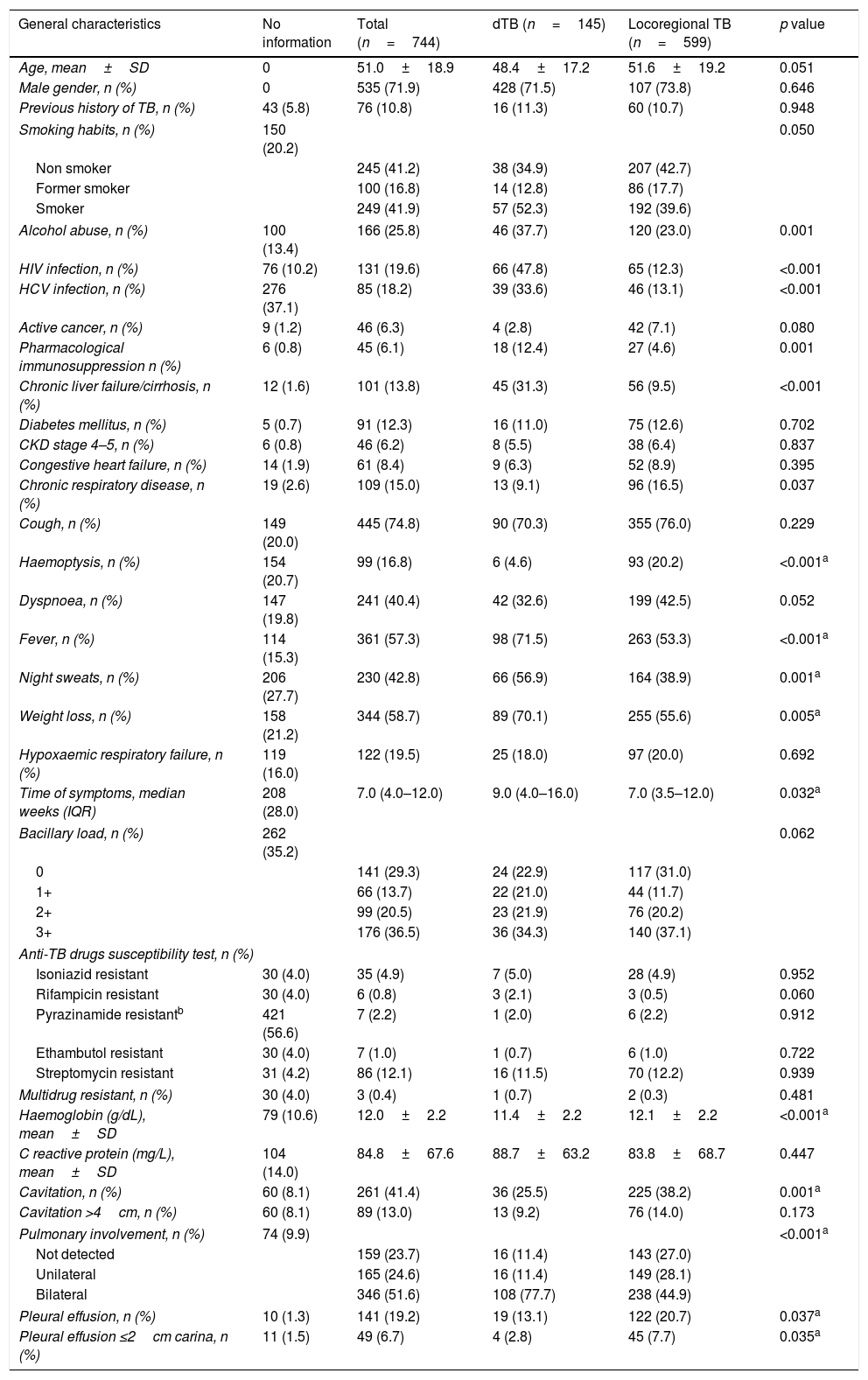
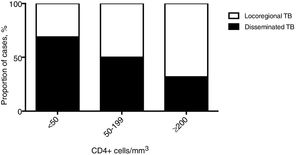
Clinical featuresPatients with dTB showed increased rates of smoking and were more frequently affected by chronic liver failure or cirrhosis (either related to alcohol abuse or HCV infection) and immunosuppression due to HIV co-infection or immunosuppressive treatment (Table 1). HIV co-infection was the most common comorbidity, but the association with dTB was only observed for patients with CD4+ T-cells counts below 200cells/mm3 (Fig. 2). In contrast, chronic respiratory disease was more common in patients with locoregional TB, which comprised 533 (89%) cases with single respiratory (pulmonary and/or pleural) involvement. Regarding clinical presentation, the constitutional symptoms fever, night sweats and weight loss were more commonly present in the dTB group, while haemoptysis were less frequent in this group. A longer time between the onset of symptoms and the diagnosis was reported for dTB patients, which were also associated with lower haemoglobin values, compared to those with locoregional disease. Radiological manifestations differed between groups, with dTB patients usually showing bilateral infiltrates, though cavitation and pleural effusion were found less frequently.
Identification of risk factors for dTBA multivariate logistic regression model was used to predict the occurrence of dTB. Patients were categorized by age groups (<35, 35–49, 50–64 and ≥65 years) and the following continuous variables were transformed into binary factors using the indicated thresholds: haemoglobin <12g/dL, period of time until cultural conversion >8 weeks,16 duration of symptoms until diagnosis ≥12 weeks. The cut-off point for the duration of symptoms was based on the Youden index criteria, when giving equal weight to sensitivity and specificity.17
The independent risk factors for dTB were the following: pharmacological immunosuppression, HIV co-infection, chronic liver failure or cirrhosis, time of symptoms ≥12 weeks, bilateral lung involvement, absence of haemoptysis, absence of dyspnoea, the occurrence of weight loss and night sweats (Table 2). The accuracy of the model was confirmed with an area under the ROC curve of 0.821 (95% IC 0.784–0.859).
Correlation between selected variables and dTB (univariate analysis) and independent risk factors for dTB (multivariate analysis).
| dTB associated factors | n | Crude OR | CI 95% | p value (UA) | Adj. OR | 95% CI | p value (MA) |
|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | |||||||
| 35–49 | 225 | 1.3 | 0.8–2.2 | 0.230 | |||
| 50–64 | 154 | 0.8 | 0.5–1.5 | 0.556 | |||
| ≥65 | 201 | 0.6 | 0.4–1.1 | 0.116 | |||
| Smoking habits (previous or current) | 349 | 1.4 | 0.9–2.1 | 0.135 | |||
| Alcohol abuse | 166 | 2 | 1.3–3.1 | <0.001* | |||
| HIV infection | 131 | 6.6 | 4.3–10.0 | <0.001* | 5.1 | 3.1–8.3 | <0.001* |
| HCV infection | 85 | 3.4 | 2.1–5.5 | <0.001* | |||
| Active cancer | 46 | 2.7 | 0.9–7.7 | 0.062 | |||
| Pharmacological immunosuppression | 45 | 3 | 1.6–5.6 | 0.001* | 5.6 | 2.8–11.3 | <0.001* |
| Chronic liver failure/cirrhosis | 101 | 4.3 | 2.8–6.8 | <0.001* | 2.3 | 1.4–4.1 | 0.002* |
| Chronic respiratory disease | 109 | 0.5 | 0.3–0.9 | 0.029* | |||
| Haemoptysis | 99 | 0.2 | 0.1–0.4 | <0.001* | |||
| Absence of haemoptysis | 3.2 | 1.3–8.4 | 0.012* | ||||
| Dyspnoea | 241 | 0.7 | 0.4–0.9 | 0.042* | |||
| Absence of dyspnoea | 1.9 | 1.2–3.1 | 0.011* | ||||
| Fever | 361 | 2.2 | 1.5–3.3 | <0.001* | |||
| Night sweats | 230 | 2.1 | 1.4–3.1 | 0.001* | 1.7 | 1.1–2.7 | 0.020* |
| Weight loss | 344 | 1.9 | 1.2–2.9 | 0.004* | 1.8 | 1.1–2.9 | 0.015* |
| Symptoms for ≥12 weeks | 178 | 2 | 1.3–3.1 | 0.001* | 2.3 | 1.4–3.8 | 0.002* |
| Rifampicin resistance | 6 | 4.2 | 0.8–20.9 | 0.082 | |||
| Haemoglobin <12g/dL | 313 | 2 | 1.4–2.9 | <0.001* | |||
| Cultural conversion >8 weeks | 175 | 0.7 | 0.4–1.3 | 0.283 | |||
| Cavitation | 261 | 0.5 | 0.3–0.7 | 0.001* | |||
| Cavitation >4cm | 89 | 0.6 | 0.3–1.2 | 0.136 | |||
| Bilateral pulmonary involvement | 346 | 4.1 | 2.7–6.4 | <0.001* | 4.4 | 2.8–7.1 | <0.001* |
| Pleural effusion | 141 | 0.6 | 0.3–0.9 | 0.039* | |||
| Pleural effusion ≤2cm from carina | 49 | 0.3 | 0.1–0.9 | 0.043* | |||
Definition of abbreviations: dTB: disseminated tuberculosis; OR: odds ratio; UA: univariate analysis; Adj OR: adjusted odds ratio; MA: multivariate analysis; HIV: human immunodeficiency virus; HVC: hepatitis C virus.
In the study cohort, a total of 156 (21%) patients died during the first 6 months of treatment. Mortality was higher for dTB patients, when compared to locoregional TB (36% vs 20.8%, respectively), although no statistically significant difference was found in the present cohort (p>0.05). Similarly, but not even reaching statistical significance, a longer time until culture conversion was observed in dTB patients (22.5±6.9 vs 11.2±8.0 weeks).
DiscussionRisk factors for dTB may be empirically known, but have not been systematically modelled in a cohort of patients. In turn, this may contribute to a delay in the diagnosis of dTB, which further aggravates the disease.1 Disseminated TB remains a public health concern in sub-Saharan countries,1 mostly driven by the syndemic of HIV.18 In Portugal, disseminated disease is a rare presentation, accounting for only 1.3% of all TB cases notified in the national report of 2014.9 Contrasting with this figure, we describe a high frequency of dTB (19.5%) in the present cohort, which, to the best of our knowledge, supports the largest analysis of its kind in a European population. Although this represents a much higher proportion compared to the general population, previous studies have also shown higher prevalence of dTB in hospitalized patients.7 This might be explained by the more aggressive diagnostic workup that is usually performed in hospitals,5 thus improving detection rates of disseminated disease. On the other hand, the hospital where the study was based serves a large urban area and is a reference centre for treatment of several comorbid conditions related to/with higher risk for dTB, particularly HIV, increasing the proportion of co-infected patients compared to that reported in the regional monitoring system.19 Nevertheless, 10.2% subjects were not tested for HIV in our sample. Although mandatory after TB diagnosis, we believe that the frequency of non-tested cases was influenced by clinician's personal judgement, comprising essentially older patients without any known risk factors for HIV co-infection.
Both HIV infection and pharmacological immunosuppression, including transplant recipients, were main risk factors for dTB in the present study. The importance of cell-mediated immunity was further illustrated by the inverse correlation found between the CD4+ T cells count in HIV patients and the frequency of cases of dTB. Previous studies have highlighted the prognostic value of T cells count in this context1,20 and have shown that blood culture may be a valuable tool for diagnosing dTB in HIV co-infected patients and that mycobacteremia is often a sign of bad prognosis.1 Thus, early recognition and treatment are likely to be important measures and, given the longer time for culture conversion observed, future randomized controlled trials are needed to determine the optimal duration of treatment in these particular cases. Retrospective reviews have previously shown higher relapse rates in HIV-1 infected patients treated for 6 months compared to those treated for longer21 and that ≥8 months of rifamycin-containing treatment might be required for optimal treatment outcomes.22
Additionally, the existing studies reveal that dTB prevalence is also higher in diabetics, alcohol abusers, patients with chronic liver disease, cases of malnutrition and those undergoing hemodialysis.5,24 Remarkably, in this study patients with diabetes and advanced chronic kidney disease did not show increased risk for dTB. However, we report a significant association with chronic liver failure/cirrhosis, either caused by alcohol abuse or HCV infection. The liver seems to have a central role in the clearance of bacteria from the bloodstream, through the hepatic reticuloendothelial system, mainly by Kupffer cells.25,26 Furthermore, laboratory evidence highlights a reduction of T cell function and chemotaxis in patients with liver cirrhosis.27 These data may thus explain why dTB is a common complication in patients with advanced liver disease.28
The time between the onset of symptoms and the diagnosis was higher in the dTB group, highlighting the problem of diagnostic delay due to unspecific clinical presentation.24 This might have led to more advanced disease, with dTB patients reporting higher rates of constitutional symptoms, namely fever, night sweats and weight loss. Studies that took place in low resource regions and with poor access to healthcare described symptoms lasting for up to 52 weeks.29,30 Like a previous report,31 our data suggest that the delay in TB treatment might also increase the likelihood of M. tuberculosis dissemination, although this hypothesis should be further explored in future controlled studies. In fact, dTB patients had more frequently bilateral lung involvement, which means that local disease progression may precede systemic progression. However, our study did not show that the delay in diagnosis had an impact on mortality. Longer evolution of the disease in the dTB group may be also associated to a decline in haemoglobin level, which is often a marker of iron deficit.32 Iron deficiency itself has been associated with impaired cellular immunity and reduced ability to control infection.32,33
Chronic respiratory disease, which is a known risk factor for pTB,34,35 was less frequent in patients with disseminated disease, as were a few classic features of respiratory involvement (dyspnoea, haemoptysis, presence of cavitation and pleural effusion). However, 40% of dTB in our sample had a miliary lung involvement. A review study revealed that radiographic aspects of miliary TB are present in about 29% of the cases of dTB,7 but frequency may reach 70% with HIV co-infection.4
Due to the retrospective design of the study, the selection and analysis of certain variables were limited by lack of information. Still, our results highlight relevant data. For instance, alcohol intake was sometimes reported in a qualitative manner, as regular consumption, or abuse, which may be affected by the personal perspective of the clinician. But, importantly, this variable allowed us to etiologically classify the chronic liver failure. Another limitation inherent to this study lies in the fact that this was a hospital sample, which might not be representative of the outpatient patients or accurately reflect the TB national panorama. Finally, the inclusion of miliary TB criteria in the definition of dTB may have affected the sampling, due to the low sensitivity and specificity of pulmonary radiological presentation.7,36 This limitation was waived by independent review of chest X-rays by two clinicians.
ConclusionsThe non-specificity of the signs and symptoms may limit a timely diagnosis of dTB and contribute to this globally growing problem. The results observed in this study revealed predictors that may aid the diagnosis of disseminated disease, enhancing the early onset of treatment and consequently improving the clinical outcome. Patients who are immunocompromised, have chronic liver failure or cirrhosis, and longer duration of symptoms, specially fever, night sweats and weight loss, are at higher risk of developing dTB. A more systematic approach should be offered to these patients, particularly to HIV patients with under 50 CD4+ cells/mm3, for whom performing blood cultures and bone marrow or liver biopsy sampling may enhance diagnostic yield of disseminated forms of disease. Future studies will be required to determine if these cases would benefit of longer treatment regimens.
Conflicts of interestThe author has no conflicts of interest to declare
We acknowledge the support of Dr. Ana Maria Correia, Dr. Eduardo Coutinho and Olga Barbosa in providing us access to the regional TB surveillance system data. HNB acknowledges the receipt of research scholarships from Bolsa D. Manuel de Mello and the Portuguese Society for Pneumology. NSO acknowledges FCT IF/00474/2014. The MS lab is financed by FEDER – Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 – Operational Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274). MS and NSO are FCT Associate Investigators. The funding agencies had no role in the design of the manuscript.