Patient selection criteria and experimental interventions of randomized controlled trials may not reflect how things work in practice. The aim of this study was to describe the characteristics of chronic obstructive pulmonary disease (COPD) patients undergoing an inpatient pulmonary rehabilitation program (PRP) and the correlates of success.
MethodsRetrospective database review of 975 consecutive patients transferred from acute care hospitals after an acute exacerbation (group A: 14.6%) or admitted from home (group B: 75.4%), from 2010 to 2017. Patients were also divided according to the associated registered main diagnosis: COPD (group 1: 30.6%); COPD and respiratory failure (group 2: 51.7%); COPD and obstructive sleep apnea (group 3: 17.6%). Baseline correlates of post-PRP changes in six minute walking test (6MWT) were also evaluated.
ResultsGlobal Initiative for Chronic Obstructive Lung Disease stages 3 and 4 were the most commonly represented in group 2 (p=0.0001). Comorbidity Index of all patients was 3.9±1.8. The overall in-hospital mortality rate was 1.3% (5.6% vs 0.6%, in groups A and B, respectively; p=0.0001). Hypertension, cardiac diseases and obesity were observed in 65.2, 52.2 and 29.6% of patients, respectively. Post-PRP 6MWT increased in all groups. Age, male gender, airway obstruction and baseline 6MWT were correlated with a post-PRP 30 meter increase in 6MWT.
ConclusionConfirming data of literature, this real-life study shows the characteristics of COPD patients undergoing an inpatient PRP with significant improvement in exercise capacity, independent of whether in stable state or after a recent exacerbation or of the associated main diagnosis.
Chronic obstructive pulmonary disease (COPD) is associated with several comorbidities contributing to different phenotypes, with related mortality rates and reduced physical activity.1,3
Pulmonary rehabilitation programs (PRPs) are main components of the comprehensive management of these patients. After a PRP, patients, either in stable state or following an acute exacerbation of COPD (AECOPD), report improvements in their symptoms, health-related quality of life (HRQL), and exercise tolerance.4,5 This is also true when complex comorbidities and different phenotypes are present.6–8 However, there is no accepted selection criteria for PRPs for these patients.9
We wondered whether characteristics of patients and results of PRPs reported in randomized controlled trials (RCTs) and meta-analyses could also be observed in real health services. Therefore, the aim of this real-life retrospective observational study was to evaluate the characteristics of COPD patients undergoing an inpatient PRP and to identify the predictors of its success (if any).
MethodsThe protocol was approved (2164 CE, December 5, 2017) by the Ethical Committee of the Istituti Clinici Scientifici Maugeri IRCCS, Pavia, Italy. All patients at admission to the Scientific Institute of Montescano gave informed consent to the scientific use of their data.
Study participantsThis retrospective review was conducted on a database of patient discharge records, available from January 2010 to September 2017. The study population included all COPD patients consecutively admitted for inpatient PRPs to the Pulmonary Rehabilitation Unit of the Scientific Institute of Montescano, a referral center for pulmonary rehabilitation, diagnosis and management of obstructive sleep apnoea syndrome (OSAS),10 and care of chronic patients including prescription of home noninvasive ventilation.11 COPD patients were either transferred from acute care hospitals, after an AECOPD as described elsewhere5,8 or admitted from home on referral by their general practitioners.
Diagnosis and severity of COPD were confirmed by spirometry according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.1 At the time of admission, all patients were in stable conditions as assessed by the absence of worsening in symptoms, i.e. no change in the patient's dyspnea, cough, and/or sputum beyond day-to-day variability which would have been sufficient to warrant a change in management and stability in blood gas values (e.g. no respiratory acidosis (pH>7.35) compared to the conditions reported at home or on discharge from the referring hospital. All patients received their regular treatment with inhaled bronchodilators with or without inhaled steroids according to current guidelines for their disease stage.1
For the purpose of this study, respiratory failure was defined as the presence of at least one of the following conditions: (1) long-term oxygen therapy (LTOT); (2) arterial oxygen tension (PaO2) <55.0mmHg at rest; (3) a 4% drop in pulse oximetric oxygen saturation (SpO2) to <90% during the six minute walking test (6MWT).
According to the International Classification of Sleep Disorders, OSAS12 was defined as the presence of five or more obstructive respiratory events (apnea, hypopnea, or respiratory effort-related arousal) per hour of sleep plus daytime or nighttime symptoms of OSAS; alternatively, as the presence of 15 or more obstructive respiratory events per hour of sleep.
Pulmonary rehabilitation programOur standard inpatient multidisciplinary PRP includes the optimization of drug therapy, education, nutritional programs, and psychosocial counseling, when appropriate and five weekly sessions for 4 weeks, consisting of:
- 1.
Supervised incremental exercise training according to Maltais et al.13 until performing 30min continuous cycling at 50–70% of the maximal load calculated on the basis of the baseline 6MWT according to Luxton et al.14
- 2.
Abdominal, upper, and lower limb muscle activities lifting progressively increasing light weights (0.30–0.50kg), shoulder, and full arm circling.15
A multidisciplinary team consisting of chest physicians, nurses, physical therapists, dieticians, and psychologists offer care.
MeasurementsThe following data were reported from patients’ discharge data records:
- •
Diagnosis. According to the recorded associated main diagnosis, patients were divided into three groups: group 1: COPD; group 2: COPD and respiratory failure; group 3: COPD and OSAS.
- •
Provenance. According to the recorded provenance, patients were divided into: group A: transferred from acute care hospitals after an AECOPD; group B: admitted from home.
- •
Rehabilitation center length of stay (LoS) and modality of discharge (home, transfer to acute care hospital, hospital death).
- •
Demographics (age, gender), anthropometrics (body mass index: BMI16), and biochemical data.
- •
Reported number and diagnosis of comorbidities, according to the Cumulative Illness Rating Scale (CIRS) including the Comorbidity Index (CI) and the Severity Index (SI).17
- •
Dynamic lung volumes, assessed according to the American Thoracic Society (ATS) guidelines18 by means of a body plethysmograph (MasterScope Body Jeager-Wuerzburg, Germany) or a water-sealed spirometer (EOS Biomedin, Padova, Italy). The predicted values according to Quanjer were used.19 Lung function data were available in 769 patients.
- •
Arterial blood gases, measured by means of an automated analyzer (Radiometer 800Flex, Copenhagen, Denmark) on samples from the radial artery with the patients in the sitting position for at least 1h. Patients under LTOT were assessed under their usual inspiratory oxygen fraction. Data for arterial blood gases were available for 701 patients.
- •
Exercise tolerance, assessed by means of the 6MWT according to accepted standards.20 The best of two consecutive performances (2h apart) conducted under pulse oximetric monitoring, in a 30-m long and 3-m wide corridor under quiet conditions and without distractive stimuli was recorded for analysis. At the beginning and at the end of walking, subjective sensation of both dyspnea and leg fatigue was assessed, but not reported in the database, by means of the modified Borg scale.21 The minimal clinically important difference (MCID) of 6MWT following exercise training in COPD has been reported to be at least a 30m increase.20 Data of 6MWT were available for 424 patients.
Descriptive statistics are reported as mean (SD) for continuous variables and as numbers (n) and percentage frequency (%) for discrete variables. Between-group comparisons were carried out by one-way analysis of variance (ANOVA) and by the Chi-square test for continuous and categorical variables. An overall statistically significant difference in group means revealed by the ANOVA was followed-up by post hoc analysis (Tukey–Kramer test). Within-group comparisons were carried out by paired t-test.
The association between baseline 6MWT and demographic, anthropometric, physiological, and clinical variables (age, gender, BMI, CI, SI, serum glucose and hemoglobin (Hb), Forced Expiratory Volume at 1 second (FEV1)/Forced Vital Capacity (FVC) ratio, arterial carbon dioxide tension (PaCO2), and PaO2) was assessed by multiple linear regression analysis. The choice of these potential predictors was motivated by the need to reduce the problem of multicollinearity, arising when two or more independent variables are highly correlated, leading to unreliable statistical inferences.
Finally, the association between the presence of an improvement in the 6MWT greater than the MCID and the same variables described above, including also baseline 6MWT, was assessed by logistic regression.
All statistical tests were two-tailed and statistical significance was set at p<0.05. Missing data were excluded from analysis (complete-case analysis approach). All analyses were carried out using the SAS/STAT statistical package, release 9.4 (SAS Institute Inc., Cary, NC, USA).
ResultsOut of 3311 patients admitted during the study period, there were 975 (29.4%) patients with COPD undergoing the inpatient PRP. Of these, 142 (14.6%) were transferred from acute care hospitals (group A) and 833 (85.4%) were admitted from home (group B) (Table 1). Groups 1–3 included 30.7, 51.7, and 17.6% of patients, respectively (Table 2).
Demographic, anthropometric, physiological, clinical characteristics of all patients and according to provenance: mean (SD).
| All subjects (N=975) | Group A (N=142) | Group B (N=833) | p-Value | |
|---|---|---|---|---|
| LoS (days) | 27.3 (12.6) | 31.9 (14.4) | 26.5 (12.1) | 0.0001 |
| Age (years) | 71.1 (10.5) | 71.7 (10.2) | 71.0 (10.5) | 0.44 |
| CI | 4.0 (1.8) | 4.4 (1.8) | 3.9 (1.8) | 0.003 |
| SI | 1.8 (0.3) | 1.9 (0.3) | 1.8 (0.3) | 0.0003 |
| BMI (kg/m2) | 27.3 (7.3) | 24.3 (5.9) | 27.7 (7.4) | 0.0001 |
| FEV1/FVC (%) | 52.8 (15.1)† | 53.2 (17.3) | 52.8 (14.9) | 0.82 |
| FEV1 (L) | 1.33 (0.64)† | 1.23 (0.54) | 1.35 (0.65) | 0.09 |
| FEV1 (% of predicted) | 54.7 (21.5)† | 49.4 (17.8) | 55.4 (21.9) | 0.012 |
| FVC (L) | 2.53 (0.92)† | 2.37 (0.87) | 2.55 (0.93) | 0.09 |
| FVC (% of predicted) | 80.8 (22.6)† | 73.6 (19.4) | 81.8 (22.9) | 0.001 |
| PaCO2 (mmHg) | 39.0 (6.6)^ | 39.5 (11.4) | 38.9 (5.6) | 0.42 |
| PaO2 (mmHg) | 68.6 (10.0)^ | 66.1 (10.3) | 69.0 (10.0) | 0.010 |
| pH | 7.43 (0.03)^ | 7.44 (0.05) | 7.43 (0.03) | 0.045 |
Group A: patients transferred from acute care hospitals; Group B: patients admitted from home.
LoS, length of stay; CI, Comorbidity Index; SI, Severity Index; BMI, body mass index; FEV1, Forced Expiratory Volume at 1 second; FVC, forced vital capacity; % of pred, percent of predicted values; PaCO2, arterial carbon dioxide tension; PaO2, arterial oxygen tension;
p-Values refer to between-group comparisons. †N=769 ^N=701.
Demographic, anthropometric, physiological, and clinical characteristics of patients according to recorded associated main diagnosis. Mean (SD) or n (%) as specified.
| Group 1 (N=299) | Group 2 (N=504) | Group 3 (N=172) | p-Value | |
|---|---|---|---|---|
| Males:females | 213: 86 | 353:151 | 136: 36 | 0.07 |
| Age (years) | 69.8 (12.3)‡ | 72.8 (9.2)^ | 68.3 (9.5) | 0.0001 |
| BMI (kg/m2) | 24.9 (5.2)‡* | 26.7 (7.4)^ | 33.0 (7.0) | 0.0001 |
| BMI≥30kg/m2, n (%) | 49 (16.4) | 130 (25.8) | 110 (63.9) | <0.0001Γ |
| BMI<18.5kg/m2, n (%) | 31 (10.4) | 53 (10.5) | 1 (0.6) | 0.0001Γ |
| FEV1/FVC (%) | 53.2 (14.0)†* | 49.3 (16.3)^ | 60.0 (11.0) | 0.0001 |
| FEV1 (L) | 1.44 (0.66)‡* | 1.09 (0.54)^ | 1.71 (0.59) | 0.0001 |
| FEV1 (% of predicted) | 58.8 (21.1)‡* | 46.6 (20.7)^ | 66.1 (16.6) | 0.0001 |
| FVC (L) | 2.70 (0.94)‡ | 2.25 (0.85)^ | 2.86 (0.87) | 0.0001 |
| FVC (%predicted) | 86.4 (22.1‡ | 74.1 (22.9^ | 86.8 (18.5 | 0.0001 |
| GOLD I, n (%) | 44 (17.2) | 27 (7.6) | 30 (18.8) | |
| GOLD II, n (%) | 117 (45.7) | 114 (32.3) | 104 (65.0) | |
| GOLD III, n (%) | 74 (28.9) | 125 (35.4) | 25 (15.6) | |
| GOLD IV, n (%) | 21 (8.2) | 87 (24.6) | 1 (0.6) | 0.0001Γ |
| PaO2 (mmHg) | 73.9 (8.9)‡ | 62.4 (7.7)^ | 74.2 (8.0) | 0.0001 |
| PaCO2 (mmHg) | 37.4 (5.0)‡ | 40.6 (7.9)^ | 37.7 (4.4) | 0.0001 |
| pH | 7.43 (0.03) | 7.43 (0.03) | 7.44 (0.03) | 0.17 |
| PaO2≥80mmHg, n (%) | 57 (23.9) | 9 (2.7) | 27 (20.3) | |
| 55≤PaO2<80mmHg, n (%) | 177 (74.4) | 276 (83.6) | 105 (78.9) | |
| PaO2<55mmHg, n (%) | 4 (1.7) | 45 (13.6) | 1 (0.8) | 0.0001Γ |
| PaCO2≥45mmHg, n (%) | 13 (5.5) | 74 (22.4) | 8 (6.0%) | |
| PaCO2<45mmHg, n (%) | 225 (94.5) | 256 (77.6) | 125 (94.0) | 0.0001Γ |
Group 1: COPD; Group 2: COPD and respiratory failure; Group 3: COPD and OSAS.
†p<0.01 for the comparison Group 1 vs Group 2; ‡p<0.001 for the comparison Group 1 vs Group 2; *p<0.001 for the comparison Group 1 vs Group 3; ^p<0.001 for the comparison Group 2 vs Group 3; Γp-Value from Chi-square test.
Demographic, anthropometric, physiological, and clinical characteristics of patients according to provenance are described in Table 1. Patients of group A had longer LoS, lower BMI, suffered from more comorbidities as assessed by CI and SI, respectively, and lower levels of PaO2.
The LoS were different across groups of diagnosis (group 1: 26.7 (13.3), group 2: 28.7 (12.8), group 3: 24.3 (10.1) days; p=0.0002), with a statistically significant difference between group 2 and group 3 (p=0.001).
Characteristics of patients according to the recorded associated main diagnosis as well as their distribution across BMI, the GOLD classes, and levels of arterial blood gases are described in Table 2. As expected, obese patients were significantly more represented in group 3, whereas only about 10% of patients of both groups 1 and 2 were underweight. Data of lung function and arterial blood gases were available for 769 and 701 patients, respectively. Patients of group 2 were significantly older and more represented in GOLD stages III and IV, showed lower mean levels of PaO2 and higher mean levels of PaCO2 than the others.
Mean biochemical data of patients at admission are shown in Table 3.
Biochemical data: mean (SD).
| Serum level | All subjects | Group 1 | Group 2 | Group 3 | p value |
|---|---|---|---|---|---|
| Creatinine (mg/dL) | 1.0 (0.4) | 1.0 (0.4) | 1.0 (0.4) | 1.0 (0.4) | 0.93 |
| Glucose (mg/dL) | 102 (33) | 95 (19)†* | 102 (33)^ | 114 (46) | 0.0001 |
| Cholesterol (mg/dL) | 182 (41) | 183 (41) | 181 (40) | 183 (45) | 0.73 |
| Triglycerides (mg/dL) | 115 (56) | 112 (54)* | 112 (55)^ | 130 (62) | 0.0008 |
| Hb (g/L) | 13.1 (1.8) | 13.2 (1.8)† | 12.9 (1.9)^ | 13.5 (1.6) | 0.0002 |
Group 1: COPD; Group 2: COPD and respiratory failure; Group 3: COPD and OSAS.
†p<0.05 for the comparison Group 1 vs Group 2; *p<0.01 for the comparison Group 1 vs Group 3; ^p<0.001 for the comparison Group 2 vs Group 3.
Distribution among groups of associated diagnosis of reported components of CIRS and assessed comorbidities are shown in Table 4. Patients of group 1 suffered from less CI and SI than the other groups. Heart (valvular disease and chronic heart failure) and cardiovascular diseases had a similar prevalence in patients of all groups. Hypertension was present in more than half the patients of all groups; it was more prevalent in patients of groups 2 and 3 than in patients of group 1. The overall endocrine diseases reported, including diabetes, occurred in more than 60% of patients of all groups, and as expected occurred in the vast majority of patients of group 3. As also shown in Table 4, hypercholesterolemia and hypertriglyceridemia as assessed at admission was common across all groups, whereas hyperglycemia had a significantly greater prevalence in group 3. Heart (p=0.007) and cardiovascular (p=0.0003), as well as kidney (p=0.0001) diseases occurred more significantly among patients transferred from hospitals (group A), whereas obesity was significantly more common among patients in group B (p=0.0001).
Hospital LoS, components of CIRS, and referred or assessed comorbidities: mean (SD) or n (%) as specified.
| All subjects | Group 1 | Group 2 | Group 3 | p-Value | |
|---|---|---|---|---|---|
| Comorbidity Index | 3.98 (1.83) | 3.61 (1.80)† | 4.08 (1.81)‡ | 4.34 (1.82) | 0.0001 |
| Severity Index | 1.83 (0.33) | 1.77 (0.33)* | 1.84 (0.32)Γ | 1.88 (0.31) | 0.0008 |
| Heart, n (%) | 509 (52.2) | 151 (50.5) | 273 (54.2) | 85 (49.4) | 0.44 |
| Cardiovascular, n (%) | 434 (56.4) | 130 (50.8) | 219 (62.0) | 85 (53.1) | 0.36 |
| Hypertension, n (%) | 636 (65.2) | 175 (58.5) | 328 (65.1) | 133 (77.3) | 0.0002 |
| Upper GI tract, n (%) | 262 (26.9) | 81 (27.1) | 125 (24.8) | 56 (32.6) | 0.14 |
| Lower GI tract, n (%) | 231 (23.7) | 66 (22.1) | 120 (23.8) | 45 (26.2) | 0.60 |
| Liver, n (%) | 190 (19.5) | 58 (19.4) | 87 (17.3) | 45 (26.2) | 0.039 |
| Kidney, n (%) | 155 (15.9) | 54 (18.1) | 73 (14.5) | 28 (16.3) | 0.40 |
| Urinary, n (%) | 314 (32.2) | 101 (33.8) | 159 (31.5) | 54 (31.4) | 0.78 |
| Musculoskeletal, n (%) | 592 (60.7) | 176 (58.9) | 315 (62.5) | 101 (58.7) | 0.50 |
| CNS, n (%) | 188 (19.3) | 60 (20.1) | 99 (19.6) | 29 (16.9) | 0.67 |
| Endocrine, n (%) | 683 (70.1) | 184 (61.5) | 355 (70.4) | 144 (83.7) | 0.0001 |
| Obesity, n (%) | 289 (29.6) | 49 (16.4) | 130 (25.8) | 110 (64.0) | 0.0001 |
| Hypercholesterolemia, n (%) | 298 (32.8) | 101 (33.7) | 160 (31.8) | 59 (34.1) | 0.79 |
| Hypertriglyceridemia, n (%) | 78 (8.0) | 25 (8.3) | 34 (6.7) | 19 (11.0) | 0.20 |
| Hyperglycemia, n (%) | 218 (22.4) | 43 (14.5) | 116 (23.1) | 59 (34.1) | <0.0001 |
Group 1: COPD; Group 2: COPD and respiratory failure; Group 3: COPD and OSAS.
GI, gastrointestinal; CNS, central nervous system.
†p<0.001 for the comparison Group 1 vs Group 2; *p<0.01 for the comparison Group 1 vs Group 2; Γp<0.01 for the comparison Group 1 vs Group 3; ‡p<0.001 for the comparison Group 1 vs Group 3; ^p<0.001 for the comparison Group 2 vs Group 3.
The overall hospital mortality rate was 1.3% (5.6% vs 0.6%, in groups A and B, respectively, p=0.0001; 0.7, 2.2, and 0.0% in groups 1, 2, and 3, respectively, p=0.048), mainly due to patients with respiratory failure transferred from acute care hospitals after an AECOPD. These patients also accounted for most discharges to acute care hospitals (6.5% of all patients; 15.5% vs 4.9%, in groups A and B, respectively, p=0.0001; 4.7, 9.1, and 1.7% in groups 1, 2, and 3, respectively, p=0.001). AECOPD was the main cause of death (13 patients), or of transfer to acute care hospitals (63 patients) together with other complications (unavailable data). No death or transfer to acute care hospitals was due to the activities of PRP nor was any adverse effect observed during sessions.
Patients who died were older (79.8±6.1 vs 71.0±10.5 years, p=0.003), suffered from more severe comorbidities as assessed by means of the SI (2.0±0.3 vs 1.8±0.3, p=0.020), and at admission showed lower levels of PaO2 (51.7±12.7 vs 68.7±9.9mmHg, p=0.0001) and higher levels of PaCO2 (53.9±38.1 vs 38.8±5.6mmHg, p=0.0001) than patients discharged alive.
Besides death or transfer to acute care hospitals, from the available data dropouts from PRPs were due to voluntary withdrawal (23 patients).
Values of 6MWT before and after the PRPs were available only for 424 patients. Missing data were mainly due to lack of inclusion into database, and to a lesser extent to dropouts from PRPs or impossibility of performing the 6MWT at discharge. Changes in 6MWT after the PRPs are shown in Tables 5a and 5b. At admission, patients transferred from acute care hospitals showed reduced 6MWT as compared to patients admitted from home. Patients in both groups improved 6MWT after PRP but patients transferred from acute care hospitals showed a greater improvement in 6MWT than patients of group B (Table 5a). Patients of group 2 showed significantly lower baseline values of 6MWT than the other groups. However, patients of all groups significantly improved their exercise tolerance (Table 5b). The patients who improved their 6MWT by more than the MCID were 48.4% of all patients without any significant difference between groups: 45.5, 50.3, and 48.5% in groups 1, 2, and respectively, p=0.76. There was no difference in success of PRPs between male and female patients.
Pre- to post-changes in 6MWT in all patients and according to provenance of patients: mean (SD).
| All subjects (N=424) | Group A (N=48) | Group B (N=376) | p-Value | |
|---|---|---|---|---|
| 6MWT, admission (m) | 310.1 (108.3) | 256.4 (101.9) | 317.0 (107.3) | 0.0002 |
| 6MWT, discharge (m) | 345.0 (98.1) | 309.6 (94.2) | 349.7 (97.8) | 0.016 |
| Δ6MWT (m) | 30.0 (48.0)* | 48.5 (44.4)* | 27.6 (48.0)* | 0.012 |
Group A: patients transferred from acute care hospitals; Group B: patients admitted from home.
*p<0.0001 testing the null hypothesis Δ6MWT=0 (within subject).
Pre- to post-changes in 6MWT according to recorded associated main diagnosis.
| Group 1 (N=129) | Group 2 (N=205) | Group 3 (N=90) | p-Value | |
|---|---|---|---|---|
| 6MWT admission (m) | 341.7 (105.4)† | 273.8 (102.5)^ | 347.7 (98.7) | 0.0001 |
| 6MWT discharge (m) | 363.8 (103.1)† | 319.4 (91.3)^ | 375.3 (91.5) | 0.0001 |
| Δ6MWT (m) | 24.9 (39.3)* | 33.3 (54.3)* | 30.2(44.8)* | 0.40 |
Group 1: COPD; Group 2: COPD and respiratory failure; Group 3: COPD and OSAS.
*p<0.0001 testing the null hypothesis Δ6MWT=0 (within subject); †p<0.001 for the comparison Group 1 vs Group 2; ^p<0.001 for the comparison Group 2 vs Group 3. Abbreviations as in the text.
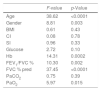
Out of all considered potential predictors, multiple regression analysis showed a significant association with baseline 6MWT only for age, male gender, serum Hb, FEV1/FVC, FVC, % predicted, and PaO2 but not for BMI, CI, SI, serum glucose, and PaCO2. All significant associations were positive except for age (inverse association). Detailed results from multiple regression analysis are reported in Table 6a.
Finally, the results of logistic regression analysis modeling the probability of an improvement in the 6MWT greater than the MCID using the same predictors as above and including baseline 6MWT are given in Table 6b. The only significant predictors were age, gender, FEV1/FVC (borderline), and baseline 6MWT. In particular, for a 1 year increase in age and 1 meter increase in baseline 6MWT, there were respectively an 8 and 1.8% increase in the probability of an improvement in the 6MWT greater than the MCID after PRP.
Logistic regression results. Dependent variable: presence of an improvement in 6MWT>30m.
| p-Value | Odds ratio estimates | |||
|---|---|---|---|---|
| Point estimate | 95% confidence limits | |||
| Age | 0.0001 | 0.919 | 0.880 | 0.960 |
| Gender (F vs M) | 0.009 | 0.335 | 0.148 | 0.760 |
| BMI | 0.87 | 1.005 | 0.949 | 1.063 |
| CI | 0.34 | 0.837 | 0.579 | 1.209 |
| SI | 0.39 | 0.381 | 0.042 | 3.469 |
| Glucose | 0.62 | 0.997 | 0.985 | 1.009 |
| Hb | 0.97 | 1.004 | 0.796 | 1.267 |
| FEV1/FVC % | 0.05 | 1.028 | 1.000 | 1.057 |
| FVC % pred | 0.39 | 1.008 | 0.990 | 1.027 |
| PaCO2 | 0.10 | 0.944 | 0.881 | 1.011 |
| PaO2 | 0.26 | 1.025 | 0.982 | 1.069 |
| 6MWT admission | <0.0001 | 0.982 | 0.977 | 0.988 |
CI, Comorbidity Index; SI, Severity Index; BMI, body mass index; FEV1, Forced Expiratory Volume at 1 second; FVC, forced vital capacity; % of pred, percent of predicted values; PaCO2, arterial carbon dioxide tension; PaO2, arterial oxygen tension; Hb, Hemoglobin; F, female; M, male.
This real-life study shows the characteristics of patients with COPD undergoing an inpatient PRP resulting in significant improvement in exercise capacity independent of provenance or associated main diagnosis, confirming in a “real-life” setting the results of RCTs and meta-analyses.
The demographic characteristics of an OSAS occurrence in our patients are those commonly reported for COPD patients undergoing PRPs.7,8,22,23 Underweight was reported only in 10% of patients independent of associated respiratory failure. In agreement with Greening et al.,24 in our patients BMI did not influence their baseline 6MWT or their response to PRP. Obesity is observed in 17% of patients with AECOPD25 and, like our study, it has no effect on the percentage of patients achieving the MCID of 6MWT after PRP.26,27
More than a third of patients admitted to our PRP suffered from severe airway obstruction as assessed by distribution of patients in GOLD stages III or IV (Table 2). In our study, neither the associated diagnosis of respiratory failure, lower PaO2, or higher PaCO2 levels negatively influenced the success of PRP, confirmation for a larger number of patients than other previous reports.28 Therefore, the results of our study support the guideline suggestions to perform PRPs in almost all COPD stages.1,4,29
Among COPD patients, type 2 diabetes mellitus and hypertriglyceridemia showed a prevalence between 16.8–28.5 and 19.7%, respectively.30 In the study by Griffo et al.,7 hypercholesterolemia was less prevalent (22.4%), whereas the occurrence of diabetes mellitus (17.4%) was similar to the reported prevalence of diabetes mellitus in our study.7
Hypertension is observed in 28.5–64.7%7,30 of COPD patients and occurred in more than 60% of our patients. Reported heart and cardiovascular diseases occurred in about half of our patients. Heart failure is reported between 12.3 and 28.3%,30 whereas in study by Griffo et al.7 chronic heart failure and cardiovascular diseases showed 11.9 and 51.9% prevalences, respectively.
In patients with COPD, the number of comorbidities can predict mortality31 and comorbidity-attributable costs were twice those directly related to COPD.32 A systematic review of literature suggested that comorbidities might also have a negative influence on PRP outcomes.33 Our study confirms in a large COPD population the results of other studies6,7,34 that comorbidities do not preclude patients with COPD from obtaining improvements in exercise capacity. Our study confirms also the findings by Crisafulli et al.6 that the number of comorbidities is not predictive of PRP outcomes.
Confirming other studies,35 we did not observe any deaths or the need to transfer patients to acute care hospitals due to PRP nor was any adverse effect observed during PRP activities. Our PRP also improved exercise tolerance in patients recovering from an AECOPD. Meta-analyses give conflicting results on the safety and the clinical benefits of PRP after an AECOPD.36,37 There is evidence that aerobic training is safe even when conducted after 72h of hospitalization for AECOPD,38 however a RCT39 reported that early rehabilitation during hospital admissions for chronic respiratory diseases did not reduce the risk of subsequent readmissions and was associated to higher 12-month mortality.
Finally, our study confirms that baseline exercise capacity and severity of airway obstruction but not comorbidities are predictors of success of PRPs in exercise tolerance as assessed by reaching the MCID of 6MWT.6,40 However, an earlier study showed that baseline 6MWT was the only variable that correlated and significantly predicted also the post-PRP changes (delta) in exercise capacity in these patients.8
Our study has several limitations. Like all retrospective studies, it suffers from missing data such as in 6MWT or PRP adherence rate and importantly, it reports only one direct outcome of PRPs, the 6MWT, without any information on HRQL, or symptoms. On the other hand, retrospective analyses of large populations describe the real life of health services. The reported comorbidity occurrence must not be considered as ‘prevalence’, because in our study, patients did not undergo specific diagnostic tests. However, our comorbidity occurrence was comparable to the prevalence observed in studies in which the comorbidities were systematically investigated.2
In conclusion, the inpatient PRP resulted in significant improvement in patient's exercise capacity independent of whether in stable state or recovering from a recent AECOPD or of associated respiratory failure or OSAS, confirming that characteristics of patients and results of PRP reported in RCTs and meta-analyses can be observed also in a routine setting.
FundingNone.
Conflicts of interestNone of the authors declare any conflict of interest with this paper.