Latent tuberculosis infection (LTBI) diagnosis in a country with a low tuberculosis burden is complicated. Since the prevalence of LTBI in second generation immigrants has not been well recognized, we conducted a cross-sectional study which aimed to explore the differences in LTBI prevalence between offspring of immigrants from high tuberculosis (TB) burden countries and those whose parents were born in countries with a low TB burden.
MethodsBetween May 2014 and April 2018 young native Israelis who were required to perform pre-occupational tuberculin skin tests (TST) (medical and paramedical personnel or teaching assistants of immigrants from high TB burden countries) and who had a TST result of 10mm and above were tested for QuantiFERON-TB In Tube (QFT-GIT). Statistical comparisons were made between second generation immigrants and those with both parents from a low TB burden country.
ResultsOf 102 patients, 71 were born to parents both of whom were from low-risk countries, 14 to one parent from a high-risk country and 17 to parents both of whom were from a high-risk country. The odds ratio for LTBI was 4.5 (95% CI, 1.2...17.2; p=0.03) if both parents were born in a high-risk country compared to both parents being from a low-risk country and 4.01 (95% CI, 1.12...14.3; p=0.03) higher compared to persons for whom at least one parent was born in a low-risk country.
ConclusionThe risk for latent TB is significantly higher in second generation immigrants if both parents were born in a high-risk country. IGRA should be considered before treatment to patients with a positive TST if at least one parent was born in a low-risk country in order to confirm LTBI.
Latent tuberculosis infection (LTBI) identification is a challenging task especially in countries with a low tuberculosis (TB) burden. Health care personnel undergo baseline TB screening in many countries. Guidelines recommend testing for M. tuberculosis (Mtb) infection by either a tuberculin skin test (TST) or an interferon-gamma release assay (IGRA) for those without documented evidence of prior LTBI or TB disease.1 Since TST is more available, easy to perform and cheaper than IGRA, it is more widely used. Although TST sensitivity is high (95%) the specificity is relatively lower (85%) than the QuantiFERON TB Gold In Tube (QFT-GIT; Cellestis Limited, Carnegie, Victoria, Australia) test (99%) due to its cross-reactivity with the bacille Calmette...Gu..rin (BCG) vaccination or exposure to nontuberculous mycobacteria.2...4 Current guidelines recommend that individuals 5 years or older at low-risk for Mtb infection and disease progression in whom diagnostic testing for LTBI is warranted, should undergo a second diagnostic test if the initial test was positive.1,5 Therefore, IGRA is frequently used to identify false-positive results of TST in order to reduce over-diagnosis and unnecessary treatment of LTBI.6...9
TST should be more cautiously interpreted in countries with a low incidence of tuberculosis. Immigrants from high TB burden countries make this interpretation more difficult resulting in many questions regarding TB transmission to natives or to second generation immigrants. Furthermore, the TB burden is different for each country and its transmission varies between different communities.10 Nevertheless, transmission of TB from immigrants to native populations has been found to be low. In Norway, using molecular fingerprint patterns, little influence of M. tuberculosis transmission by immigrants from high-incidence countries to the receiving low-incidence country population was found over a period of twelve years.11
Treatment decisions of LTBI for immigrants from high TB burden countries is still challenging.12...14 Long-term development of active TB appears higher in immigrants with a positive IGRA compared to TST which has reduced LTBI treatment of negative IGRA results.15 An area of uncertainty is the prevalence of LTBI in second generation immigrants from high TB burden countries. In a study of LTBI among 9th grade school children in Norway, second generation immigrants were defined as: born in a Western country with one or both parents of non-Western origin. The risk for TST...15mm was 1.53 higher than children of Western-born parents. However, the QuantiFERON TB Gold (QFT-G) test positivity risk was similar in both groups.16
Active TB was found to be higher among the second generation of immigrants. In one study, a higher rate of tuberculosis was seen among second generation migrants compared to native residents in Berlin.17 In another study, TB in the second-generation was seen in 3.7...15.1% of all immigrants from high TB burden countries to the Netherlands.18 This calls for a better understanding of TB transmission in the second generation of immigrants from countries with a high burden of tuberculosis.
Israel is a country with a very low TB burden (incidence less than 10 per 100,000 of the population)19 and most cases of TB are in people born out of Israel according to the Israeli Ministry of Health (IMH).20 In Israel, recent immigrants from countries with a high TB burden and persons who are pre-employment, volunteering or studying in a place with a high risk for TB infection (e.g., medical centers, nursing homes, working with immigrants from high TB countries) must undergo a TST. If the two step TST>10mm, the patient must be referred to a TB clinic in order to consider further testing and treatment with 4 months rifampin or 9 months isoniazide.
The aim of this study is to explore the differences of LTBI in young native Israelis (18 years old and above), among those who were born to parents from low TB burden countries and second generation immigrants from high TB burden countries. Our hypothesis is that LTBI is more prevalent in offspring of immigrants from countries with a high TB burden than others whose parents were born in countries with a low TB burden. Since at-risk pre-occupational LTBI investigation in Israel is performed by TST, we offered the QFT-GIT to individuals with a TST of 10mm or more and considered positive QFT-GIT as an indicator for LTBI.
MethodsIn this cross-sectional study we recruited civilians and soldiers who were required by the IMH to have TST (TUBERSOL, 5 TU in 0.1ml, Medici Medical Ltd, Israel) before at-risk occupations. In Israel, soldiers who are designated to work as dental assistants or teaching assistants of immigrant children from countries with a high TB burden as well as civilians from the medical or paramedical professions are required to have TST prior to the potential exposure. In this study, healthy individuals, who had never been exposed to patients with TB, and who had a pre-occupational TST of 10mm or above (two steps if indicated) according to their healthcare providers were offered testing by the QFT-GIT. The whole-blood assay of QFT-GIT was performed according to the manufacturer...s instructions (Cellestis, Ltd., Victoria, Australia) at the Laboratory of Pulmonary and Allergic Diseases,Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel. Other inclusion criteria were: signing an informed consent, age 18 years or older and native Israeli men and women. We included in the study adult children of at-least one parent that was born in a high TB burden country (incidence rate of at least 40 per 100,000)19 as well as -those whose parents were both born in low TB burden countries. Participants were considered ..úlow-risk..Ñ if both parents were born in a low-risk country for TB, ..úmedium-risk..Ñ if one parent was born in a high-risk country and ..úhigh-risk..Ñ if both parents were born in a high-risk country.
Exclusion criteria were: prior exposure to patients with tuberculosis (active or past), working/volunteering with high-risk populations or in a medical institution, living in a high-risk country, immunocompromised patients, HIV carriers, pregnancy, acute illness or less than 4 weeks since a live vaccination such as HBV or MMR.
Study participants were asked to fill a demographic and risk-stratification questionnaire that included the country of birth of each parent, known exposure to persons with active tuberculosis, working or volunteering in a medical institute or having travelled to high risk countries for more than a month. Their BCG vaccination record and physical examination for a BCG vaccination scar were noted. The study was approved by the Institutional Review Boards of Sheba Medical Center, Tel-HaShomer, Israel and of the Israel Defense Forces (IDF) Medical Corps.
Statistical analysisComparison between risk groups (Table 1) was performed using ANOVA for continuous parametric variables, and the Kruskal...Wallis rank-sum test for non-parametric variables. Independence between binomial categorical variables was tested using Fisher's exact test. We looked at correlations using Pearson's test for continuous parametric variables and Spearman...s rank test for non-parametric variables. Odds ratios for evaluating risk between groups were calculated using logistic regression (Table 2). Statistical analysis was performed using R software, version 3.4.1 (R Project for Statistical Computing).
Participant...s characteristics and results.
| Risk groupa | Total | Low | Medium | High | p-Value | |
|---|---|---|---|---|---|---|
| No. | 102 | 71 | 14 | 17 | ||
| Age (years) | Mean (sd) | 21.1 (3.4) | 21.7 (3.5) | 19.9 (2.6) | 19.8 (2.5) | 0.005 |
| TST (mm) | Mean (sd) | 14.8 (5.0) | 14.9 (5.3) | 14.2 (3.9) | 14.7 (4.4) | 0.91 |
| Gender (%) | Female | 80 (78.4) | 51 (71.8) | 12 (85.7) | 17 (100.0) | 0.031 |
| Male | 22 | 20 | 2 | 0 | ||
| IGRA (%) | Negative | 89 (87.3) | 65 (91.5) | 12 (85.7) | 12 (70.6) | 0.065 |
| Positive | 13 | 6 | 2 | 5 | ||
Odds ratio for positive quantiferron test.
| Risk groupa | Odds ratio | 95% CI; p-value |
|---|---|---|
| High risk vs. low risk | 4.5 | 1.2...17.2; 0.03 |
| High risk vs. medium and low risks | 4.01 | 1.12...14.31; 0.03 |
| High and medium risks vs. low risk | 3.16 | 0.96...10.4; 0.057 |
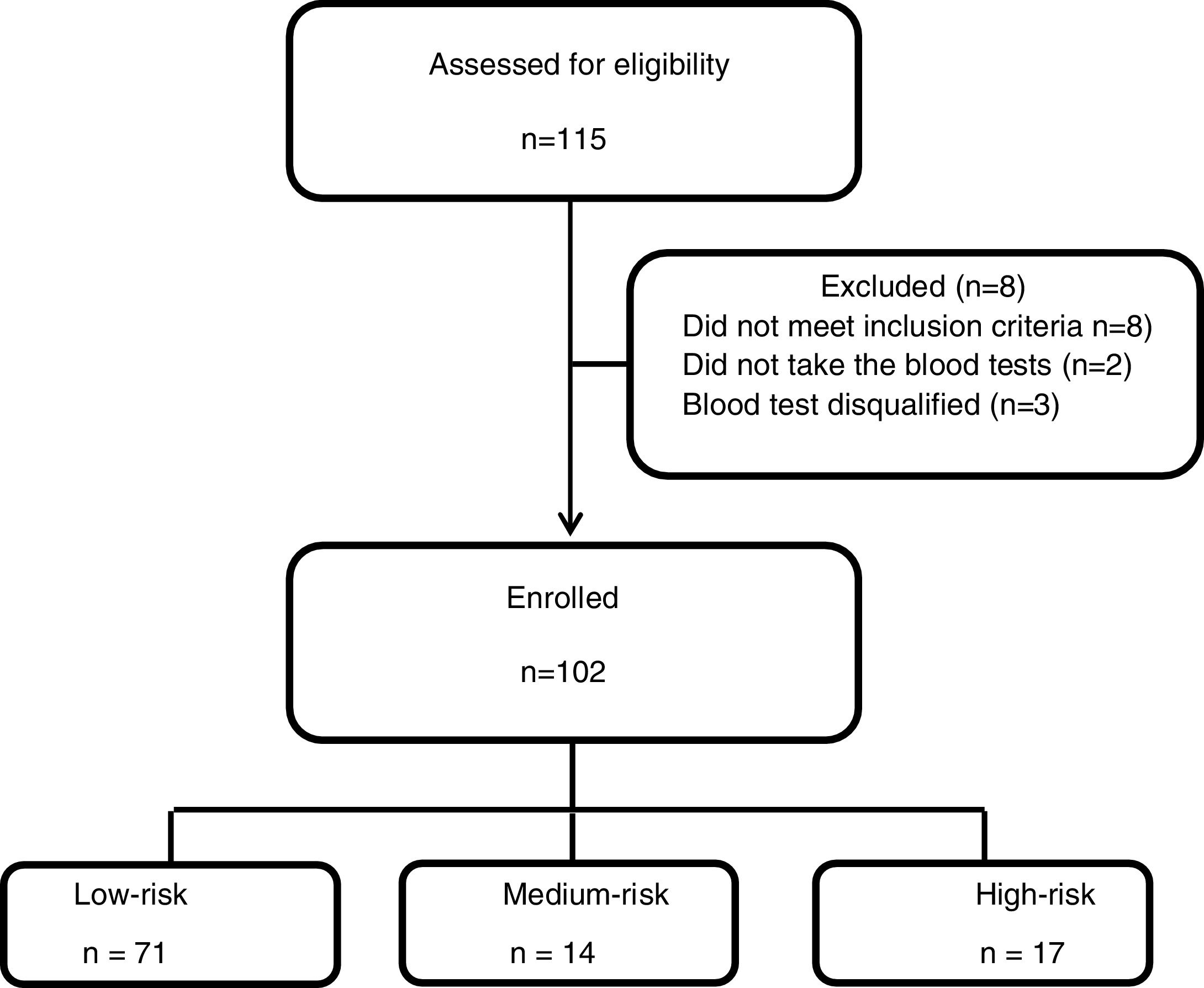
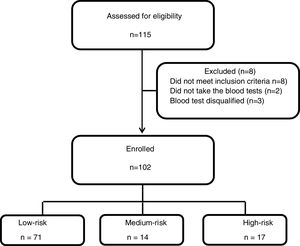
Between May 2014 and April 2018 one hundred and fifteen patients were recruited to the study, 8 patients had exclusion criteria, two participants did not take the QFT-GIT test and 3 blood tests results were technically disqualified. Of the 102 participants, 71 were ..úlow-risk..Ñ, 14 were ..úmedium-risk..Ñ and 17 were ..úhigh-risk..Ñ (Fig. 1). Table 1 summarizes participant's characteristics and results. The mean age was 21.1 years (..3.4), and was slightly higher in the low-risk group (p=0.005); this, however, is not clinically significant. There were a higher proportion of women (80.4%) and the high-risk group was exclusively females. The TST results were between the study cut-off of 10mm and 35mm with a mean of 14.8 (.. 5) without significant differences between the groups (p=0.91).
The overall differences between the 3 risk groups were not statistically significant for a positive QFT-GIT result (p=0.065). However, statistically significant differences in the prevalence of LTBI was found between the high and the low-risk groups, with an odds ratio [OR] for a positive test result of 4.5 (95% confidence interval [CI], 1.2...17.2; p=0.03) for the high-risk group (Table 2). When we combined the medium and the low-risk groups, and compared them to the high-risk group, the statistically significant difference remained, with an OR for LTBI of 4.01 (95% CI, 1.12...14.3; p=0.03) for the high-risk group. However, when we combined the high and medium groups and compared them to the low-risk group we found borderline significance for LTBI if at least one parent was born in a high TB burden country (OR: 3.16 (95% CI, 0.96...10.35; p=0.057)). Due to the small number of positive IGRA results, comparisons according to the country of origin of the parents were not justified. In the positive IGRA groups 5 participants in the high risk category were born to immigrant parents from the former USSR (2), Romania (1), Ethiopia (1) and Brazil (1). Of 8 persons in the medium risk category, one participant had a parent who had immigrated from Morocco, and another participant had a parent who had immigrated from South Africa; the other 6 participants were born to native Israelis (low risk classification).
TST and IGRAWe did not find a correlation between TST size and positive QFT-GIT results (r=0.18) for the entire group. However, we found a substantial positive correlation for positive IGRA and higher TST results (r=0.61, p=0.02) in the medium risk group. We did not find any correlation between the positive IGRA and participants with a TST of 15mm or greater (r=0.14). Also, we did not find any correlation between age or sex and either TST size or a positive IGRA results.
BCG vaccinationOnly six participants had received BCG vaccination at birth. Five were in the high-risk group, and only one of them had a positive IGRA result. One participant in the low-risk group who was born at a time when all Israeli newborns received the BCG vaccination (until 1982) had a negative IGRA result.
DiscussionLTBI rates are influenced by contact with active TB patients. Second generation immigrants from countries with a high TB burden are potentially at greater risk for LTBI than native people of the same age. However, second generation immigrants may have one immigrant parent from a high burden country or two immigrant parents from the same or different countries with high TB burdens. Furthermore, immigrant communities from different countries have different socioeconomic status which may lead to a different risk of TB transmission.10,18,21 For example, the extent of within-country transmission is much lower (about half) for the Turkish and Indonesian communities than for the Moroccan population in the Netherlands.18 These findings call for further contact investigation with a focus on differences between communities such as the number of residents in a house, living with grandparents or other family members, community activities, population density and other socioeconomic parameters. Other studies have shown that foreign patients in high-income countries, especially low socioeconomic groups are at high risk for disease progression.22,23
In this study we evaluated the prevalence of LTBI (defined by both TST of 10mm or more and a positive IGRA result) in second generation immigrants from countries with a high TB burden. We found an OR of 4.5 (95% CI, 1.2...17.2; p=0.03) for LTBI if both parents were born in a country with a high TB burden (high-risk group) compared to parents from a low TB burden country (low-risk group) that was statistically significant. The risk for LTBI remained significantly higher if we combined the medium risk group (one parent from a high TB burden country) and the low-risk group (OR, 4.01; 95% CI, 1.12...4.31; p=0.03). However, when we combined the medium and the high-risk groups, we found borderline significance for higher risk for LTBI if at least one parent was born in a high TB burden country compared to both parents from a low TB burden country (OR 3.16; 95% CI, 0.96...10.4; 0.057). These findings may indicate that TB transmission in a country with a low TB burden would require an extensive level of exposure to immigrants from high TB burden countries. A recent study showed that US-born individuals from neighborhoods with a high population density and neighborhoods at a socioeconomic disadvantage have a greater risk for TB transmission while race/ethnicity was a significant risk for the foreign born population.24 Children of two immigrant parents in our study may have a higher level of exposure (parents, relatives, neighborhood and life-style) and therefore are at a greater risk for LTBI. In Berlin, second generation immigrants had a 2.2 greater risk for tuberculosis than natives, at a younger median age (27.5 vs. 52 respectively).17 In a study among school children in Norway, although the risk for positive TST in second generation immigrants was higher (1.53) than children of Western-born parents, similar rates of positive QFT-G test were found.16 However this study is different from our study in several aspects. First, the group of second generation immigrants was defined as one or both parents from a non-Western country. Second, the burden of TB in the country of origin was not specified and third, the study population was younger (9th grade school children). In a large study of risk factors for LTBI among recruits undergoing basic army training in the US, living with a family member who was not born in the US had an OR of 13.2, 9.3 and 6 for positive TST, QFT-GIT and T-SPOT results respectively.5 Our results call for further studies in a larger population in order to better identify the risk of second generation immigrants from a high TB burden country.
In this study we did not find a correlation between the TST size and a positive QFT-GIT test for the entire study population. However, in the medium risk group a substantial positive correlation for positive IGRA and higher TST results was found (r=0.61, p=0.02). This result should be cautiously interpreted since this was the smallest group in the study (n=13). In the study of school children in Norway, the proportion of positive QFT-G tests increased with the size of the TST indurations, however, positive tests were seen for all TST sizes.16 In another study in children in the US, increasing TST size was associated with a trend towards increased rates of IGRA positivity, however, this was not seen uniformly even in children with very large TST indurations.25 Several studies in the era prior to IGRA use risk assessment questionnaires for target screening for LTBI.21,26,27 Since the induration size of the TST cannot predict positive IGRA, we believe that the risk assessment questionnaire should be updated based on larger studies using both TST and IGRA.
The major limitations of this study are the small number of participants especially of the second generation group and the higher proportion of females who were the major participants of the army occupational training courses. This may be explained by the small numbers of eligible persons for LTBI screening in Israel, and the low positive TST rate. Another obstacle for participation was the fact that IGRA blood tests were performed in many instances in a different location to that of the recruitment which reduced the number of recruited persons who were willing to travel in order to perform the blood test. The smaller number of second generation immigrants precluded a risk stratification analysis of the country of origin and other demographic factors such as years since parent's immigrations, living with grandparents, parents' divorce and travelling to high-risk countries. A general limitation of LTBI studies are the lack of a gold-standard test for M. tuberculosis infection. However the dual TST and IGRA test reduces the false-positive results.
LTBI in second generation immigrants has not yet been well studied. In our small study we found that second generation immigrants in a low TB burden country are at a significantly higher risk for LTBI only if both parents were born in a high-risk country. However, larger studies should be conducted in order to better understand TB transmission in this group and special recommendations for screening should be put in place. Since in a low TB burden country the risk of LTBI for non-immigrants is very low, an IGRA should be considered before treatment of patients with a positive TST if at least one parent was born in a low-risk country.
Funding sourceThe study was funded by a grant from the Israel Lung Association (ILA), Tel-Aviv.
CRedit authorship contribution statementD. Shlomi: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing - original draft, Writing - review & editing. I. Galor: Methodology and Supervision. A. More: Data curation and Project administration. B. Oberman: Formal analysis and Writing - review & editing. L. Fireman: Formal analysis, Investigation and Resources.
Ethics committee approvalThe study was approved by the Institutional Review Boards of Sheba Medical Center, Tel-HaShomer, Israel and of the Israel Defense Forces (IDF) Medical Corps.
Clinical trials no. NCT02073669.
Conflicts of interestThe authors have no conflicts of interest to declare.
The study was funded by a grant from the Israel Lung Association (ILA), Tel-Aviv.