Granulomatosis with Polyangiitis (GPA), which was formerly named Wegener's Granulomatosis (WG) is a systemic disease characterized by necrotizing granulomatous inflammation and vasculitis that primarily involves upper and lower respiratory tract, as well as kidneys. Diagnosing GPA on the basis of transthoracic fine needle aspiration (TFNA) may be problematic, as it can be misdiagnosed as cancer. We describe a patient with a probable GPA which was originally diagnosed as malignancy, but who responded to lung cancer chemotherapy.
A granulomatose com poliangeíte (GPA), previamente denominada granulomatose de Wegener, é uma doença sistémica caracterizada por inflamação granulomatosa necrotizante e vasculite que envolve principalmente o trato respiratório superior e inferior, bem como os rins. O diagnóstico de GPA com base em biópsia por agulha fina (BAF) transtorácica pode ser problemático, levando ao diagnóstico incorreto de cancro. Os autores descrevem o caso de um paciente com provável GPA, inicialmente diagnosticado como cancro do pulmão, que curiosamente respondeu à quimioterapia para cancro do pulmão.
Granulomatosis with Polyangiitis (GPA), more frequently called Wegener's Granulomatosis (WG) is a systemic disease characterized by necrotizing granulomatous inflammation and vasculitis that primarily involves upper and lower respiratory tract, as well as kidneys. It can sometimes present as solitary or multifocal pulmonary lesions, without signs of extrapulmonary involvement.
Diagnosing GPA on the basis of transthoracic fine needle aspiration (TFNA) may be problematic, as it can be misdiagnosed as cancer. We describe a patient with GPA originally diagnosed as malignancy.
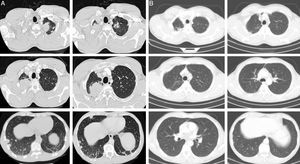
Case historyA 45-year-old man, 20 pack-year smoker, diagnosed with stage IV non-small cell lung cancer (NSCLC), by TFNA, underwent 6 cycles of chemotherapy with carboplatinum and gemcitabin, and complete remission was observed after 3 cycles (Fig. 1). At that time the cytological studies were reviewed, the diagnosis of NSCLC was maintained and antineutrophil cytoplasmic antibodies (ANCA) performed were negative. He was kept under surveillance by his Pulmonary Oncology team.
Chest CT at the time of diagnosis (A) and 3 months after chemotherapy (B). (A) Multiple nodular lesions in both lungs. The bigger lesions present in the apices. (B) Resolution of the nodular lesions after 3 cycles of chemotherapy. Some fibrotic sequelar changes can be seen in both apical regions.
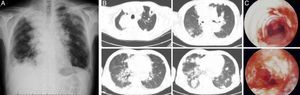
After an asymptomatic period of 15 months, with no signs of recurrence, he started having exertional dyspnea, dry cough, anorexia and weight loss (3kg over a month). Physical examination was unremarkable. Chest X-ray showed multiple nodules and masses in both lungs some of which exhibited cavitation, as confirmed by Chest CT (Fig. 2A and B).
(A) and (B) Chest radiograph (A) and CT (B) at the time of symptom relapse (15 months after chemotherapy), show multiple irregularly shaped nodules and masses, some of which exhibiting cavitation. (C) Bronchoscopic features evidencing multiple ulcerating lesions and superficial vascularization, along the whole tracheobronchial tree.
At this time a TFNA of a nodular lesion was repeated, which showed cellular debris, compatible with tumoral necrosis.
One week later, the patient developed oral ulcers and purpuric rash of lower limbs and external ear, as well as petechiae in the soft palate. Immunologic study revealed anti-neutrophilic cytoplasm antibody (cANCA) of 1/300 for proteinase 3 (PR3)>200. Urinalysis revealed active sediment with proteinuria and eritrocituria. Rigid bronchoscopy showed ulcerated lesions along the tracheobronchial tree (Fig. 2C).
Bronchial biopsies proved extensive necrosis and vasculitis, and there were no features of a neoplastic nature. A skin biopsy was also performed, revealing leucocytoclastic vasculitis.
The patient was started on methylprednisolone 1g IV od 3 days, followed by cyclofosfamide 100mg PO od and prednisolone 1mg/kg/d.
After a period of clinical and radiological improvement, respiratory failure developed requiring invasive mechanical ventilation and transfer to ICU unit. Repeated bronchoalveolar lavage revealed Klebsiella pneumonia and Mycobacterium intracellulare infection requiring therapy with imipenem and rifampin, clarithromicin and ethambutol, while keeping IV cyclophosphamide. Progressive disease was observed, with posterior development of nephrotic syndrome and renal failure. Implemented rescue therapy with plasmaferesis and rituximab, continuing with cyclophosphamide and IV immunoglobulins. Despite all the different approaches the patient did not survive.
DiscussionGPA is a vasculitis of unknown etiology first recognized as a separate entity by Friedrich Wegener in 1930, it is a granulomatous inflammation involving the respiratory tract, and necrotizing vasculitis affecting predominantly the small-sized vessels.
It affects a wide age range, with peak incidence between 6th and 7th decades. Sex-specific incidence rates do not provide a clear indication for uneven gender distribution. Its reported annual incidence ranges between 2.1 and 15 per million.1 cANCA reacting to PR3 is very (but not totally) specific of GPA, it is found in approximately 90% of patients with active generalized GPA and 60% of active limited GPA.2
A definite histological diagnosis of GPA requires coexistence of vasculitis, granuloma and necrosis although these features are only found in a minority of specimens. Only 1 or 2 of these features may be found, rendering a suggestive biopsy, in which the diagnosis is then supported by clinical criteria. Histopathologic documentation of granulomatous involvement of the respiratory tract is not explicitly required.3 Therefore, the diagnosis of GPA depends on a combination of clinical, pathologic, and serologic features.
Our case reports a probable localized GPA, interpreted as stage IV NSCLC. Since the patient only had pulmonary disease and the lesions were suggestive of metastatic lesions, ANCA were not initially tested. However, it is well known that cANCA sensitivity is as low as 60–70% in limited GPA, making its diagnosis more difficult.4
Lung nodules are the most common pulmonary manifestation of Wegener's granulomatosis and occur in approximately 40–70% of patients. Nodules are usually multiple and bilateral and occur without a zonal predilection. The size of Wegener's granulomatosis nodules varies, most commonly measuring between 2 and 4cm but ranging from a few millimeters to 10cm. The spectrum of chest CT findings is broad, ranging from nodules and masses to ground glass opacity and lung consolidation. When presented in the form of nodules, it can be easily mistaken for primary or metastatic malignancies, lung abscesses, or septic infarcts.5
Solitary or multifocal involvement of the lung by GPA without evidence of extrapulmonary disease is sometimes a difficult diagnosis due to its histopathological variability and possible misinterpretation as lung cancer. Acute inflammation, necrotic debris, reactive epithelial cells and histiocytes and atypical bronchial cells, often present in GPA, can be misinterpreted as adenocarcinoma or even as squamous cells cancer.6,7 Awasthi et al. report six cases of suspected GPA who underwent TNFA; four of them were wrongly diagnosed, one of which was a misdiagnosed squamous cell carcinoma.8 Because the positive predictive value of lung FNA ranges from 96% to 100%, a patient with a lung fine-needle aspirate diagnosed as malignant has a high probability of harboring a malignancy. However, as with any other diagnostic tests, lung FNA is subject to diagnostic pitfalls that can lead to occasional false-positive diagnoses; the false-positive rate of lung FNA has been cited as being less than 1%.9 Its diagnostic yield is worse than other methods which provide bigger samples, such as core needle biopsy, bronchial biopsy or surgical lung biopsy. Although core needle biopsy (CNB) has been shown to be an accurate means of diagnosing lung malignancies, there is relatively little information in the literature about its utility in diagnosing non-malignant conditions.
In our case, although lung cancer could not be completely excluded as a possible diagnosis, complete remission of a stage IV NSCLC made this diagnosis more unlikely. When complete remission was observed, the diagnosis of NSCLC was questioned and ANCA were performed, which were negative. Revision of TFNA cytology was also requested, as well as when generalized form of GPA was evident; giant multinucleated cells, increased nuclear/cytoplasmic ratios and irregular chromatin and irregular cell outlines were still suggestive of poorly differentiated carcinoma. In the lung cancer setting giant multinucleated cells can be present in a variable frequency; although unusual in typical adenocarcinoma these are characteristic of giant cell carcinoma and pleomorphic sarcomatoid carcinoma.
Epidermal growth factor receptor (EGFR) mutation of the cytologic sample was negative, still not helping in differential diagnosis. It was only when the disease relapsed that histologic samples could be collected and the diagnosis of GPA could be established.
GPA can emerge as a paraneoplastic syndrome, most frequently renal cell carcinoma. There is only one reported case of GPA and lung cancer, but there is no mention of whether therapy for lung cancer induced remission of GPA.10,11
Assuming ad initium the diagnosis of GPA, the cytotoxic agents used in lung cancer chemotherapy curiously led to complete remission of lesions, as well as remission maintenance for 15 months. As far as we know this is the first case reported in the literature.
The recurrence of the generalized form of GPA, although initially responsive to preconized therapy, was complicated by several infectious events and progressive respiratory failure that led to the death of the patient.
Our case describes a probable GPA in which presentation was clinically indistinguishable from lung cancer and where cytology showed, even after exhaustive review, features of undifferentiated carcinoma. It exemplifies the importance of considering alternative diagnoses, even when a particular one seems obvious.
Conflict of interestThe authors declare no conflict of interests.
Please cite this article as: S. Campainha, et al., Granulomatose com Poliangeíte inicialmente diagnosticada como Cancro do Pulmão. Rev Port Pneumol. 2012. http://dx.doi.org/10.1016/j.rppneu.2012.04.002.