In pulmonary tuberculosis, the presence of extensive residual lung lesions can be a predictor of permanent disability leading to respiratory failure.
ObjectiveTo compare functional and respiratory changes in patients with pulmonary tuberculosis sequelae who have completed treatment.
MethodThe study included patients who completed treatment within a period of 6 months (group I) and multidrug-resistant pulmonary tuberculosis patients who completed treatments of longer duration after the failure of the initial treatment (group II). We evaluated lung function by spirometry (Microlab ML 3500), the strength of respiratory muscles through the manovacuometry (MEP – maximal expiratory pressure and MIP – maximal inspiratory pressure) and the distance walked during the 6-min walk (6MWT).
ResultsTwenty-seven patients were included, 12 of whom belonged to group II, multidrug-resistant tuberculosis (MDRTB). Severe combined respiratory disorder was the most prevalent problem in group II of MDRTB; it was present in 9 patients. The MDRTB group (group II) showed significantly lower values when compared to Group I in FVC (72.06±14.95 vs 43.58±16.03% predicted), FEV1 (66.13±19.87 vs 33.08±15.64% predicted), MIP (68.40±22.78 vs 49.58±12.55cmH2O), MEP (87.20±27.30 vs 59.08±12.23cmH2O) and distance covered in 6MWT (484.21±74.01 vs 334.75±104.07m).
ConclusionPatients with multidrug-resistant pulmonary tuberculosis who have undergone multiple treatments have more severe respiratory and functional impairment than patients who have had just a single treatment.
Na tuberculose pulmonar, a presença de lesões pulmonares residuais extensas pode ser um fator preditor de invalidez permanente levando a insuficiência respiratória.
ObjetivoComparar as alterações respiratórias e funcionais em pacientes com sequelas de tuberculose pulmonar que finalizaram o tratamento.
MétodoO estudo foi realizado no Ambulatório de Tisiologia do Hospital Sanatório Partenon. Foram incluídos no estudo pacientes que finalizaram único tratamento com 6 meses de duração (grupo I) e pacientes com tuberculose pulmonar multirresistente que finalizaram tratamento de maior duração após falência aos tratamentos iniciais (grupo II). Foram avaliadas a função pulmonar através da espirometria (ML 3500 Microlab, Microlab, EUA), a força dos músculos respiratórios através da manovacuometria e a distância percorrida no teste da caminhada dos 6 minutos (TC6M). Os dados foram analisados no programa SPSS versão 13.0, sendo utilizado o teste de qui-quadrado e o t para amostras independentes. O nível de significância adotado foi de 5%.
ResultadosForam incluídos 27 pacientes sendo que 12 pertenciam ao grupo de tuberculose multirresistente. O distúrbio ventilatório mais prevalente no grupo de múltiplos tratamentos foi o padrão misto, presente em 9 pacientes. O grupo que realizou múltiplos tratamentos (grupo II) apresentou redução significativa quando comparado ao grupo I nas variáveis CVF (72,06±14,95 vs. 43,58±16,03% predito), VEF1 (66,13±19,87 vs. 33,08±15,64% predito), PImax (68,40±22,78 vs. 49,58±12,55cmH2O), PEmax (87,20±27,30 vs. 59,08±12,23cmH2O) e distância percorrida no TC6M (484,21±74,01 vs. 334,75±104,07 metros).
ConclusãoPacientes com tuberculose pulmonar multirresistente que realizaram múltiplos tratamentos apresentam comprometimentos respiratórios e funcionais maiores do que pacientes que realizaram único tratamento.
Tuberculosis (TB) continues to be a chronic infection with very high rates of morbidity and mortality. It has been estimated that each year there are 8.9 million new cases and 1.6 million deaths worldwide.1 Brazil is the 19th highest country in the world in terms of the number of TB cases and within Brazil, Porto Alegre is the state capital that has the highest incidence of the disease.1 The Brazilian TB control program is being decentralized with the progressive transfer of activities from ambulatory “TB health units” to Family Health Program (FHP) units and teams. The FHP is a new program in which teams of health professionals work in the community, linked to health units. However, cases of difficult treatment related to psychosocial problems, hepatotoxicity and worsening of health status continue to be treated in hospital.
Because there are few studies on residual sequelae of TB, the number of patients is unknown, government costs have not been evaluated and only a limited number of professionals have experience in dealing with such patients.2
The presence of extensive residual lung lesions may be a predictor of permanent disability following tissue destruction, cor pulmonale and susceptibility to opportunistic infections, leading to reduced quality of life.3 In addition, the extent of disease is one of the risk factors involved in TB mortality rates.4
The histopathological findings resulting from tuberculosis include the formation of caseous granuloma, tissue liquefaction and formation of pulmonary cavities.3
From these changes residual lesions remain in many patients, resulting in pulmonary sequelae that are characterized by impairments in the bronchial and parenchymal structure. These structural changes include broncho-vascular distortions, bronchiectasis, emphysema and fibrosis.5
Pulmonary TB can involve the airways, leading to mucosal edema, hypertrophy and hyperplasia of mucous glands, increased mucus secretion and smooth muscle hypertrophy. This affects the caliber of the airways, increases its strength and decreases airflow.6 Through the mechanism of cicatricial fibrosis there is also a reduction of total lung capacity.7
Thus, delays in the diagnosis of TB lead to the increase in lung damage and more frequent co-morbidities and impairment of quality of life.8 Treatment failure results in multidrug resistance and thus in the increase in the number of treatments, and can also be a factor in assessing the severity of the functional prognosis of patients.9,10
Post-tuberculosis patients may have limited exercise tolerance and significant disability which may affect daily activities.11 Unlike assessment of functional status in other disabling chronic diseases, there have been few studies of this kind that specifically consider TB. This study aims to evaluate and compare variables that represent the functional status in patients with sequelae of pulmonary tuberculosis who had finished treatment.
MethodsThis is a cross-sectional study with a sample selected in a non-probabilistic and intentional form, consisting of 27 patients (18 men) who were being followed up at the Ambulatory Unit of Hospital Sanatório Partenon. The period of data collection was from November 2009 to January 2010. The study included adult patients diagnosed with pulmonary tuberculosis treated as outpatients over a period of 6 months (group I) and adult patients with multiple treatments of longer duration (group II) which were also conducted in ambulatory units. Patients with tuberculosis in other organs, patients with disorders of the locomotor system with functional limitations making testing impossible, cardiovascular disease, degenerative neuromuscular diseases, and/or patients with severe hemodynamic instability were excluded from the study. Patients were selected by analyzing the records of the ambulatory units and they were contacted after their discharge. If the patient agreed to participate as a volunteer in the research, they signed the instrument of informed consent. The study was approved by the Ethics in Research Committee of School of Public Health and the Hospital Sanatório Partenon.
Chest radiographs, performed at the end of treatment, were analyzed by a radiologist and a pulmonologist. The radiological changes were ranked according to Willcox.12 The lung fields were divided into six zones, and radiographic results were classified by degree: I degree, with minimum involvement of only one zone, without cavitation; II degree, involving two or three areas or zones with cavitation; and III degree severe involvement in more than three zones with or without cavitation. The tests were administered on a single day in the following order: spirometry, manovacuometry and 6min walk test (6MWT). Information was collected through a standardized form with demographic data (age, sex, race, body mass index – BMI), clinical data, medications and smoking habits. Next, we performed a spirometry with assessment of lung function using the variables: forced expiratory volume in 1s (FEV1), forced vital capacity (FVC), FEV1/FVC ratio and peak expiratory flow (PEF). The device used was the Spirometer Microlab ML 3500 (Microlab, USA). In the test the patient was seated with elbows bent and his mouth firmly attached to the mouthpiece. The nose clip was also used to prevent air leakage. The patient was asked to perform a forced expiration starting from total lung capacity (TLC). FVC maneuvers were performed five times; the best three results were considered and then the highest one was selected as long as it did not exceed 10% of the second largest value.13
Maximum inspiratory pressure (MIP) and maximal expiratory pressure (MEP) were evaluated using the manuvacuometer.14 Between the pulmonary function tests and respiratory muscle strength, the patient rested for at least 10min. Patients had been well targeted and the tests were performed according to Guidelines for Pulmonary Function Tests.13
Finally, the physical–functional condition was assessed by the distance covered in the 6MWT. The test was conducted in a level corridor of 30m. The heart rate (HR) and oxygen saturation (SpO2) were measured by pulse oximeter Nonin Onyx 9500, (United States) and systemic blood pressure (SBP) was measured with a stethoscope and a Missouri sphygmomanometer (Brazil). For dyspnea and fatigue in the lower limbs the Modified Borg Scale was used. The respiratory rate (RR) was also measured. All these variables were measured for test control at the beginning and end of the test. The patient was asked to walk as much as possible during the six minutes and received standardized sentences of encouragement. The 6MWT was administered by visual assessment following the guidelines of the American Thoracic Society (ATS).15
The results were expressed as mean and standard deviation for data with normal distribution. For the analysis of categorical variables the chi-square was used and for the analysis of continuous variables the t-test. All tests were performed using the statistical program Statistical Package for Social Sciences (SPSS) version 13.0 for Windows. We considered the significance level of 5% (p<0.05).
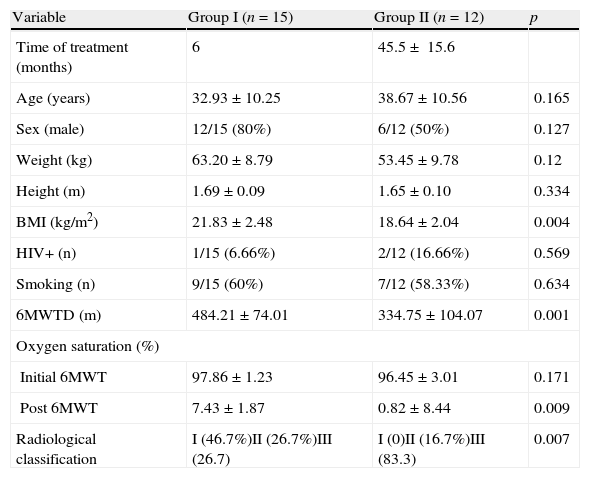
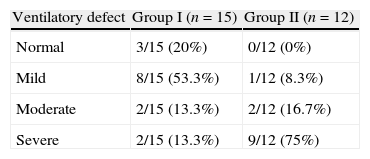
ResultsWe included 27 patients in the study and 12 were from a multiple-treatment (group II). The average time from the start of first treatment until the date of discharge at the end of treatment in group II was 45.5±15.6 months. The length of treatment of patients in group II includes the period of the treatment with scheme I; this covers treatment with rifampin, isoniazid and pyrazinamide, abandoning this treatment, subsequent attempts to treat failures of scheme I with streptomycin, ethionamide, ethambutol and pyrazinamide (including the periods of abandon and failure), and the duration of MDRTB treatment until the patient was cured (18 months). All patients in group II had culture for mycobacteria with multidrug sensitivity test, indicating failure to respond to previous treatment. There was no significant difference (p=0.634) between group I (single treatment) and group II (multiple treatments and MDRTB) in relation to the prevalence of smoking (Table 1). The groups showed no significant difference in terms of age, weight, height and sex (Table 1). Patients in group I showed radiological abnormalities with minimal involvement in 46.7% of cases (grade I according to criterion of classification). Patients in group II had severe impairment (grade III), as evaluated by radiography, in 83.7% of cases and no patient had grade I classification (Table 1). Severe combined respiratory disorder was the most prevalent in the group of multiple treatments, present in 75% of patients in group II. In the group that underwent only one treatment (group I), 20% of patients had no changes in lung function and 53.3% had mild disorder (Tables 2 and 3).
Clinical and anthropometrical sample analysis.
| Variable | Group I (n=15) | Group II (n=12) | p |
| Time of treatment (months) | 6 | 45.5± 15.6 | |
| Age (years) | 32.93±10.25 | 38.67±10.56 | 0.165 |
| Sex (male) | 12/15 (80%) | 6/12 (50%) | 0.127 |
| Weight (kg) | 63.20±8.79 | 53.45±9.78 | 0.12 |
| Height (m) | 1.69±0.09 | 1.65±0.10 | 0.334 |
| BMI (kg/m2) | 21.83±2.48 | 18.64±2.04 | 0.004 |
| HIV+ (n) | 1/15 (6.66%) | 2/12 (16.66%) | 0.569 |
| Smoking (n) | 9/15 (60%) | 7/12 (58.33%) | 0.634 |
| 6MWTD (m) | 484.21±74.01 | 334.75±104.07 | 0.001 |
| Oxygen saturation (%) | |||
| Initial 6MWT | 97.86±1.23 | 96.45±3.01 | 0.171 |
| Post 6MWT | 7.43±1.87 | 0.82±8.44 | 0.009 |
| Radiological classification | I (46.7%)II (26.7%)III (26.7) | I (0)II (16.7%)III (83.3) | 0.007 |
Group I (single treatment) and group II (multiple treatments and MDRTB).
MDRTB: multidrug-resistant pulmonary tuberculosis; HIV: human immunodeficiency virus; 6MWTD: distance walked during of the 6-min walk test. Oxygen saturation was measured by pulse oxymetry at the beginning and end of the 6min walk test.
Radiological classification criteria of Willcox. Significance: 5% (p<0.05).
Functional pulmonary testing of patients with of pulmonary tuberculosis sequelae by severity and number of treatments.
| Ventilatory defect | Group I (n=15) | Group II (n=12) |
| Normal | 3/15 (20%) | 0/12 (0%) |
| Mild | 8/15 (53.3%) | 1/12 (8.3%) |
| Moderate | 2/15 (13.3%) | 2/12 (16.7%) |
| Severe | 2/15 (13.3%) | 9/12 (75%) |
Group I (single treatment) and group II (multiple treatments and MDRTB). MDRTB: multidrug-resistant pulmonary tuberculosis.
p<0.05.
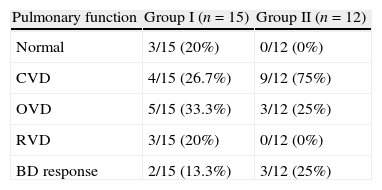
Lung function in patients with pulmonary tuberculosis sequelae by classification of the disorder and number of treatments.
| Pulmonary function | Group I (n=15) | Group II (n=12) |
| Normal | 3/15 (20%) | 0/12 (0%) |
| CVD | 4/15 (26.7%) | 9/12 (75%) |
| OVD | 5/15 (33.3%) | 3/12 (25%) |
| RVD | 3/15 (20%) | 0/12 (0%) |
| BD response | 2/15 (13.3%) | 3/12 (25%) |
Group I (single treatment) and group II (multiple treatments and MDRTB).
MDRTB: multidrug-resistant pulmonary tuberculosis; CVD: combined ventilatory disorder; ORD: obstructive ventilatory disorder; RVD: restrictive ventilatory disorder.
p<0.05.
BD response: positive response after bronchodilator aerosol treatment.
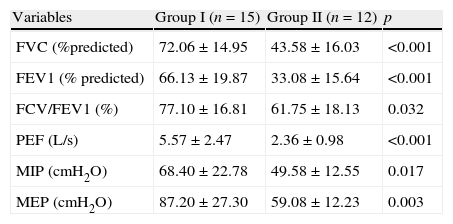
Group II, which included multiple treatments, showed significantly lower levels when compared to group I in terms of vital capacity (FVC) (72.06±14.95 vs 43.58±16.03% predicted) and FEV1 (66.13±19.87 vs 33.08±15.64% predicted) (Table 4). The analysis of the strength of respiratory muscles in group II also showed a significant reduction in MIP variables (68.40±22.78 vs 49.58±12.55cmH2O) and MEP (87.20±27.30 vs 59.08±12.23cmH2O) (Table 4). Regarding the analysis of distance covered during the 6-min walk, the group I walked 484.21±74.01m while the group II walked 334.75±104.07m, a distance significantly smaller (Table 1).
Evaluation of the lung function in patients with sequel pulmonary tuberculosis.
| Variables | Group I (n=15) | Group II (n=12) | p |
| FVC (%predicted) | 72.06±14.95 | 43.58±16.03 | <0.001 |
| FEV1 (% predicted) | 66.13±19.87 | 33.08±15.64 | <0.001 |
| FCV/FEV1 (%) | 77.10±16.81 | 61.75±18.13 | 0.032 |
| PEF (L/s) | 5.57±2.47 | 2.36±0.98 | <0.001 |
| MIP (cmH2O) | 68.40±22.78 | 49.58±12.55 | 0.017 |
| MEP (cmH2O) | 87.20±27.30 | 59.08±12.23 | 0.003 |
Group I (single treatment) and group II (multiple treatments and MDRTB).
MDRTB: multidrug-resistant pulmonary tuberculosis; FVC: forced vital capacity; FEV1: forced expiratory volume in 1s; CVF/VEF1: index tiffeneau; PEF: peak expiratory flow; MIP: maximum inspiratory pressure; MEP: maximum expiratory pressure. Significance 5% (p<0.05).
The present study demonstrates a significant increase in functional limitations in patients with pulmonary tuberculosis sequelae who had undergone multiple treatments compared to patients cured in the first treatment. Changes in lung function and large residual lesions are not common findings in patients with tuberculosis who are diagnosed early and whose treatment is appropriate and uneventful.4 Delays in the identification of cases of pulmonary TB are due to inadequate assessment of cases of symptomatic respiratory distress or delay in seeking medical attention.16 Studies have demonstrated a 7-week interval between the first visit and starting treatment and a 10–12-week interval between the onset of symptoms and starting treatment.17,18
According to guidelines for the treatment of tuberculosis, it is recommended that initial treatment be with the most effective medications and then alternative treatments used in cases of treatment failure.19 In Brazil, mainly due to higher rates of treatment noncompliance, the number of failures causing multiresistance to drugs is approximately 1.5% of treatment outcomes triggering a major public health problem.20,21 As demonstrated in our study, this problem becomes even worse when considering the functional and physical limitations that these patients may present after treatment.
The pulmonary function of patients in the group that underwent multiple treatments (group II) showed significant reductions in FVC and FEV1, compared to the group that required only one treatment (group I). Among the patients pertaining to group II, 75% had severe respiratory distress, compared to 13.3% in group I.
There are differences in the disorder of most TB sequelae. Some authors2 found that mild restrictive ventilatory disorder was the most prevalent in patients with cavitary disease, and patients without cavitation had functional normality. Others7 found a higher prevalence of obstructive disorders (68%). A study that analyzed a Brazilian population with severe obstructive pulmonary disease found that 15.7% of patients had pulmonary TB sequelae.
Another study22 concluded that TB causes chronic airflow limitation, and when it is repeated, this limitation gets worse. It would appear that the mixed disorder is the one most frequently found in patients with multidrug-resistant tuberculosis, as we found in this study.20 In our study, we found no differences between the groups in relation to the prevalence of smoking. Smoking is an important factor in the decline in lung function, but apparently was not determinant in the differences found between the groups.
The pathophysiological changes of pulmonary tuberculosis found in patients who underwent repeated treatments can cause systemic changes through motor disabilities brought on by physical deconditioning.23,24 In this study, the values obtained from analysis of BMI were lower in group II, indicating the possibility of a state of malnutrition and muscle wasting in these patients (p=0.004). Moreover, there were reductions in MIP, MEP and the distance covered during the 6MWT. The assessment of the respiratory muscle strength by measuring MIP and MEP is an important indicator of severity and exacerbation of chronic lung diseases.25 In this study, the values obtained in MIP and MEP group II showed reductions consistent with the functional changes experienced by patients.
The distance covered on the 6MWT represents the degree of functional limitation in patients with chronic pulmonary diseases of different etiologies; many of these changes can be considered a better predictor of mortality than FEV1 and nutritional status.26 Pinto-Plata and colleagues divided patients with chronic obstructive pulmonary disease into classes of 100m according to the distance walked during the test and showed that each category had significant differences in mortality outcomes.27 Moreover, in patients with severe chronic obstructive pulmonary disease, a performance of less than 300m distance walked during the test represents a mortality rate twice as high.28 In the present study, patients in group II walked on average 334.75±104.07m while the patients of group I walked on average 484.21±74.01m. Patients in group II showed a greater functional impairment as the average distance walked in 6MWT was over 100m lower, and from this we can hypothesize that they have a higher risk of early mortality.
This study demonstrated the existence of significant functional limitations in patients with sequelae of pulmonary tuberculosis. These limitations are more obvious and serious when failure occurs in initial treatment and second-line drugs are needed. These findings highlight the need for strategies to prevent treatment non-compliance and subsequent rescue regimens.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Di Naso, FC. Avaliação funcional em pacientes com sequelas de tuberculose pulmonar. S0873-2159(11)00075-4.