Interstitial lung diseases (ILDs) encompass a heterogeneous group of parenchymal lung disorders which have a significant burden on quality of life and exercise. The primary purpose of this randomised pilot trial performed in advanced ILD was to determine the feasibility and efficacy of a multidisciplinary palliative care approach (including physiotherapist, psychologist, pulmonologists, and palliative care doctors) to relieve patients’ symptoms of dyspnoea, depression measured with the Center for Epidemiological Studies-Depression (CES-D) scale and quality-of-life (QoL) at 6 and 12 months.
Matherials and MethodsFifty patients with confirmed interstitial lung disease at computed tomography (CT) scan and advanced disease were enrolled at our clinic. Patients were randomised to usual care group vs intervention group; in the intervention group, patients were scheduled to meet a physiotherapist, a psychologist, a palliative care doctor, and a pulmonologist specialized in ILD care. Data on dyspnoea, cough, quality of life and depression were recorded; patients in the intervention group were also tested to assess lower body flexibility and strength.
ResultsBoth groups showed a worsening in dyspnoea during the time course of the trial, but the Borg scale was less in the intervention group at 6 and 12 months. A similar trend was observed also for the CES-D scale. No differences were observed for the other scales.
ConclusionsA multi-disciplinary palliative care intervention in patients with advanced fibrosing interstitial lung disease is feasible and effective.
Trial registrationNCT02929966 on ClinGovTrial.
Interstitial lung disease (ILDs) encompass a heterogeneous group of parenchymal lung disorders.1 Moreover, it is challenging to predict disease progression: an unidentified proportion of patients develops an advanced phenotype, which eventually leads to a decline in lung function, respiratory insufficiency, and death.2 Idiopathic pulmonary fibrosis (IPF) is, by definition, an advancing disease, and it is also the most extensively studied one. However, other ILDs that may develop an advanced phenotype include connective tissue disease-related ILDs (CTD-ILDs),3 ILD related to chronic sarcoidosis,4 chronic hypersensitivity pneumonitis (cHP),5 and idiopathic non-specific interstitial pneumonia (iNSIP).6 Data regarding health-related quality of life (QoL) in patients with fibrosing ILDs are lacking, but the impaired QoL of patients with fibrosing ILDs is undeniable.7 Interstitial lung diseases’ course is characterized by severe motor activity and exercise limitations due to the exertional dyspnoea and fatigue,8,9 resulting in reduced quality of life.9,10 Early palliative care has been advocated by American Thoracic Society11 in any patients with chronic or advanced respiratory diseases, but only 13.7% of patients with IPF received a formal palliative care consultation.12 To date, many pharmacological and non-pharmacological therapies are available to relieve dyspnoea with, according to the Literature, opioids and pulmonary rehabilitation being among the ones with stronger evidence.13 A previous pilot study14 showed that a palliative care approach is feasible, but it mainly focused on improving QoL, anxiety, or depression, while no data were available on other symptoms like dyspnoea, and the palliative care team did not include physiotherapists or psychologists.
In this feasibility randomised trial, the purpose was to determine the feasibility and efficacy of a multidisciplinary approach (including physiotherapist, psychologist, nurses, pulmonologists, and palliative care doctors) to relieve patients’ symptoms compared to the usual care. Primary outcomes were therefore the changes in dyspnoea measured with the Borg scale,15 cough measured with the VAS scale,16 depression by means of the Center for Epidemiologic Studies Depression Score (CES-D)17 as well as perceived QoL using the Maugeri Respiratory Questionnaire (MRQr) reduced form,18 at 6 and 12 months. Exploratory outcomes included mortality and the changes in physical functioning for the intervention group to explore potential mechanisms of effect.
MethodsThe study was approved by the Sant'Orsola Hospital Ethical Committee with the number 120/2016/0/Sper. All patients were informed on the present study, and they provided written informed consent at the time of enrolment.
Study designWe conducted a parallel-group randomised controlled pilot trial; an equal randomisation of 1:1 was chosen. Inclusion criteria were (1) age ≥18 years, (2) evidence on a High-Resolution Chest CT (HRCT) of fibrosing interstitial disease19 (with at least one of the following: traction bronchiectasis and honeycombing by CT scan) (3) and evidence of advanced disease, defined as the partial pressure of oxygen in arterial blood (PaO2) ≤ 60 mmHg at room air, a decline in forced vital capacity (FVC) ≥ 10% in the previous 6 months, or a GAP index (a multidimensional index consisting of Gender [G], Age [A], and two lung physiology variables [P], forced vital capacity [FVC] and diffusion lung carbon monoxide [DLCO]) at least 3.20,21 High-Resolution Chest CT was discussed during a weekly multidisciplinary meeting by a team formed by radiologists, pulmonologists, surgeons, rheumatologists and pathologists. Exclusion criteria were: diagnosis of active cancer, treatment with anti-fibrotic therapy, and ongoing palliative pharmacological treatments. Although anti-fibrotic therapy is considered the standard of care for patients with fibrosing ILD and only a fraction of patients cannot or choose not to receive these medications, we decided to recruit only patients not taking anti-fibrotic drugs, to avoid potential bias of slowed progression (See Supplementary Material for additional data). Fifty consecutive outpatients at our ILD center were enrolled between October 2016 and September 2019. Subjects were randomly assigned to two groups, based on the activation of the palliative care intervention (intervention group) or being in the usual care group. Randomisation was performed using a generated computer sequence with block of 10. An independent statistical consultant set up the web-based randomisation process to assign eligible participants to intervention or control groups by remote allocation; no one directly involved in the project had access to allocation codes. All patients were followed-up for 12 months by their regular pulmonologist who was not blinded on group allocation.
InterventionPatients in the intervention group were scheduled to meet, at least every 6 weeks, for the whole duration of the study, a physiotherapist, a psychologist, a dedicated nurse, a palliative care doctor, and a pulmonologist experienced in ILD care. In the intervention group, the visit addressed several topics which included evaluation of patient's understanding of their illness and prognosis and establishing goals of care. Medical therapy (anxiolytic and anti-depressive medications, low-dose opioids, cough depressant, oxygen titration, etc.) was also initiated or changed to ameliorate the symptoms.
Patients allocated to the intervention group also participated in a rehabilitation program consisting of breathing and motor exercises. The same physiotherapist supervised all therapeutic sessions (60 min each) during the study period; respiratory exercises consisted of breathing control techniques, and were executed in a sitting, relaxed position. Motor exercises mainly consisted of active movements of the lower limbs directed at flexibility exercise and short active stretching of quadriceps, calves, and gluteus.22,23 Those subjects who were able to maintain a standing position without incurring severe dyspnoea, fatigue, hemodynamic instability exercised with bilateral semi-squat, standing up on tiptoe. (See Supplementary Material for additional data.). Pulmonary rehabilitation programs provided to patients with interstitial lung diseases typically include a period of 3-4 weeks of daily incremental exercise training sessions.24 Indeed, our programs lasted 12 months and it can be more aptly defined as rehabilitative counselling. To this end, patients were asked to repeat the exercises they had practised at home and they were also specifically consulted about and instructed on home-based physical activity during their visits; patients were encouraged to engage in physical activity, such as walking at their own pace for > 30 minutes most days of the week. Patients attending rehabilitative sessions were under oxygen therapy. During the treatment sessions each patient was monitored via a pulse oximeter; for those subjects who manifested O2 desaturation (i.e. peripheral oxygen saturation (SpO2)< 85%),25 the O2 supply was adjusted to guarantee a peripheral oxygen saturation (SpO2) >88%.
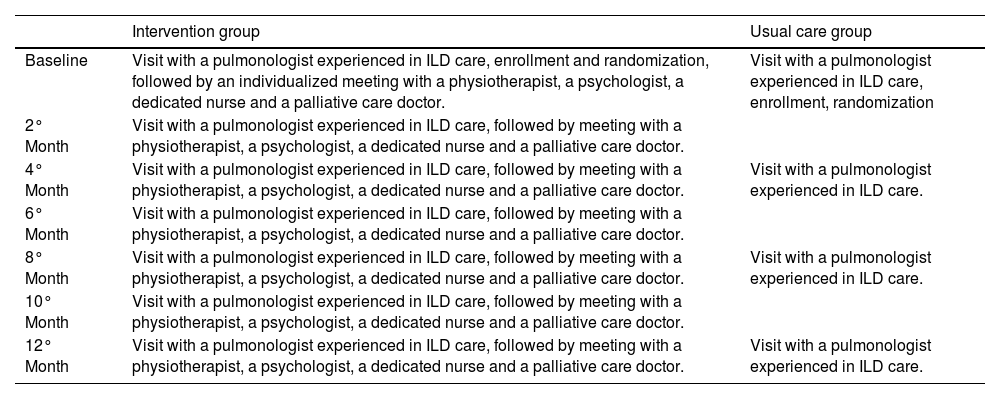
Usual carePatients in the usual care group followed the regular center schedule for medical visits (at least 3 times in a year) with a pulmonologist experienced in ILD care; medical therapy was also initiated or changed to ameliorate the symptoms. Management trails for both intervention group and usual care group during follow-up is illustrated in Table 1.
Management trails for each arm during follow up.
In both groups, at every visit, the pulmonologist recorded the intensity of perceived dyspnoea – using the Borg scale15 (score 0-10, where 10 is the worst) - and cough intensity - using the Visual Analog Scale16 (VAS) (score 0-100, where 100 is the worst). Spirometry and DLCO were performed at the baseline, using standardized references.26,27 We also obtained the Maugeri Respiratory Questionnaire (MRQr) reduced form18 for QoL impairment for chronic respiratory failure patients (score 0-28, where 28 is the worst), and the Center for Epidemiologic Studies Depression Score (CES-D)17 (score 0-60, where 60 is the worst). A brief explanation of these tools is reported in the Supplementary Material.
To explore the impact of the intervention on physical functioning over time, we examined lower body flexibility (chair sit-and-reach test)28 and strength (30-s Chair-Stand Test)29 at baseline in the intervention group. Thirty-s (30s) chair stand,28,29 consisting of counting the number of times the patient comes to a full standing position in 30 seconds. During execution of the test, the subjects were asked to place their hands on the opposite shoulder keeping their arms close to the chest. The normal range of scores in men and women was considered as predicted values.28,29 The chair sit-and-reach test was performed starting from a sitting position with a leg extended and hands reaching towards the toes.28 Measurement of the distance between extended fingers and the tip of the toe gives the result. The normal range of scores in men and women were considered the predicted ones.28 To avoid excessive fatigue and to allow a certain degree of progression, the chair sit-and-reach30 was executed before the 30-s chair stand test.
Statistical analysisTo define the sample sizes of the two groups included in the experiment, we assessed power using the Borg scale: from data collected in the 4 previous years in our clinic in this subset of patients, a mean score of 5 with a standard deviation 2.5 was estimated. In order to distinguish an average effect, change of 2 points in the scale with α=0.05 and a power of 0.8, a sample size of 25 for each treatment group was selected. Data were evaluated with an intention-to-treat analysis. Summary data are reported as mean or percentage unless otherwise stated. Homogeneity between groups was assessed to identify potential systematic differences at the baseline: chi-square independence tests were used for categorical variables, two samples independent t-tests were adopted for numerical variables after an assessment of normality and homoscedasticity assumptions. In the case of assumption violation, the Wilcoxon signed-rank test was used.
To assess the effect of the different treatments on the symptom and QoL scales, two-samples t-tests were performed to compare the mean score differences between the baseline and the follow-up (12 months) in the treatment groups. To investigate the evolution of the monitored indicators in time, we used a two-way ANOVA model with treatment group and visit time (baseline, after 6 months, and after 12 months) as factors. Adjusted p-values were produced to correct the level for multiple testing: the multivariate t distribution accounts for the correlations among the multiple hypothesis.31 The Wilcoxon test for paired samples was used to investigate if differences in the distribution of SpO2 during chair stand test, could be detected after 6 and 12 months in the intervention group. To compute the sample size for future randomised controlled trials (RCTs) aimed at assessing the statistical mean difference between the two groups for CES-D and Borg scale at 6 and 12 months (with confidence level α=0.05), the approach by Vierron and Girauderau32 was used. In the study, a p-value less than 0.05 was considered statistically relevant. The statistical analyses were carried out in the R environment.33
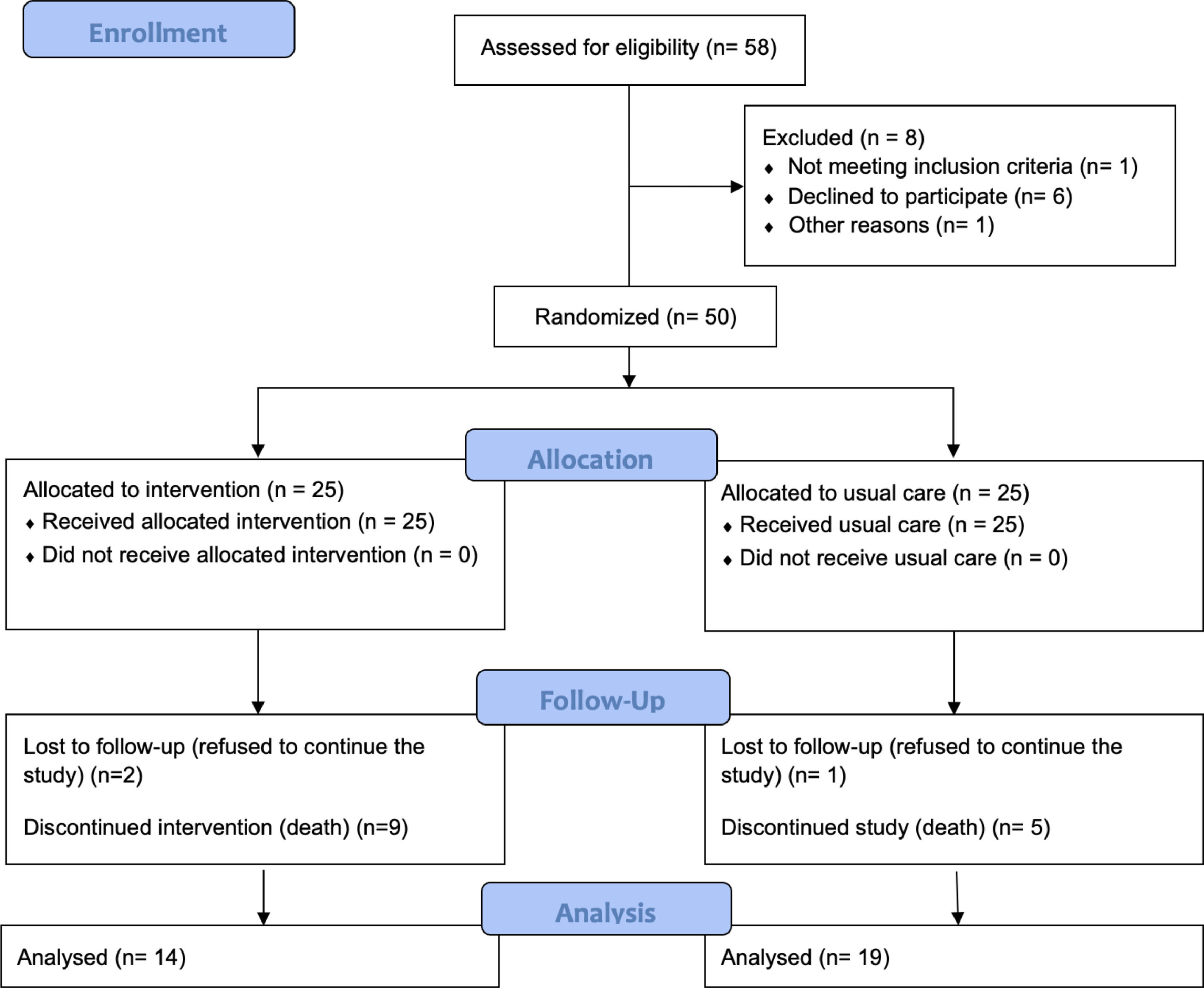
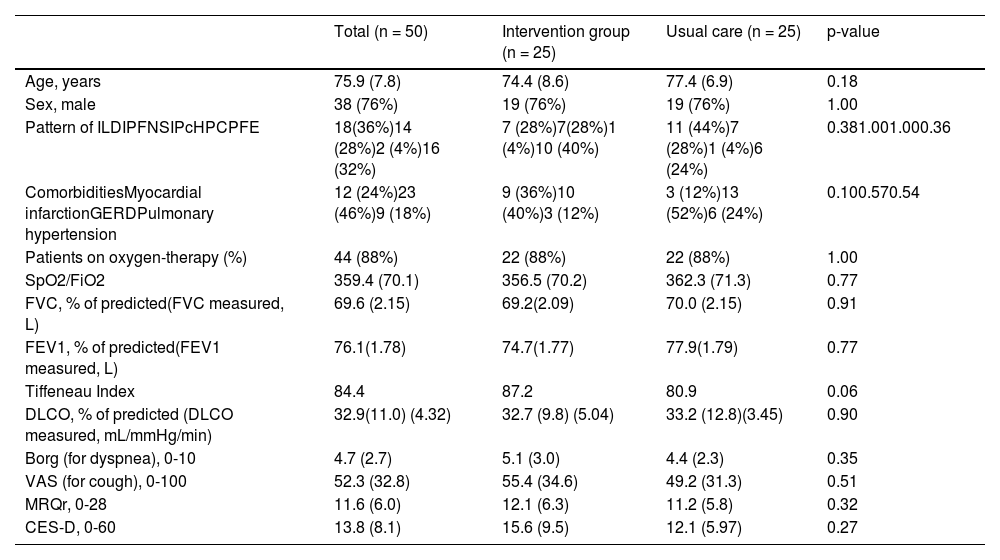
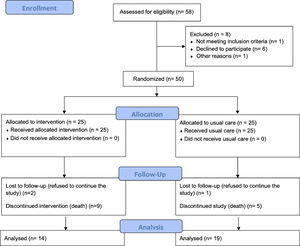
ResultsOver 3 years, 50 patients were enrolled. Fig. 1 shows the CONSORT diagram for recruitment. Eight more patients were eligible but were excluded (six refused to participate due to the distance to the hospital, one changed his mind and accepted treatment with pirfenidone, and one for unknown reasons). Most patients (n=18, 36%) had an IPF diagnosis; other diagnoses were combined pulmonary fibrosis and emphysema (CPFE, n=16, 32%), idiopathic non specific interstitial pneumonia (iNSIP, n=14, 28%) and chronic hypersensitivity pneumonia (cHP, n=2, 4%). Their other general characteristics are shown in Table 2. The number of visits per year for living patients, was 7.1±1.1 and 3.4±2.0 for the intervention group and the usual care one, respectively. The mean values of VAS for cough,22 Borg scale,21 MRQr scale,23 and CES-D scale24 were not significantly different between the groups. Fourteen patients died during the 12 months of study – 9 in the intervention group and 5 in the usual care group (no statistically significant difference) – and three more patients were lost to follow-up (2 in the intervention group and 1 in the usual care group). Therefore, all the primary outcomes were collected on all the 38 patients still alive at 6 months and for 33 patients at 12 months. No cross-over was observed.
Demographic characteristics and clinical variables at the baseline (n=50). For dichotomous variables frequency and percentage are showed and the p-value of the chi square test of independence is reported. For numerical variable mean and standard deviation are indicated and t-test p-value is reported (Wilcoxon test for CES-D and VAS).
Legend. ILD = Interstitial Lung Disease; CTD-ILDs = Connective Tissue Disease related Interstitial Lung Disease; IPF = Idiopathic Pulmonary Fibrosis; iNSIP = Idiopathic Non Specific Interstitial Pneumonia; cHP = Chronic Hypersensitivity Pneumonia; CPFE = Combined Pulmonary Fibrosis and Emphysema; BMI = Body Mass Index; GERD = Gastroesophageal Reflux Disease. SpO2 = Peripheral oxygen saturation; FiO2 = Fraction of inspired oxygen; FVC = Forced Vital Capacity; FEV1 = Forced expiratory volume in 1 second. DLCO = Diffusing capacity of the Lung for Carbon Monoxide; VAS = Visual Analogic Scale for cough; MRQr = Maugeri Respiratory Questionnaire reduced form; CES-D = Center for Epidemiologic Studies Depression Score.
We found that the intervention was feasible, with 50 of 58 eligible patients enrolling in the study (86%) and all 25 of the 25 patients randomised to the intervention receiving the intervention components (100%; see Fig. 1). Feasibility of 12-month follow-up in the intervention group was limited by patient drop out (n=2; 8%) and patient mortality (n=9; 38%).
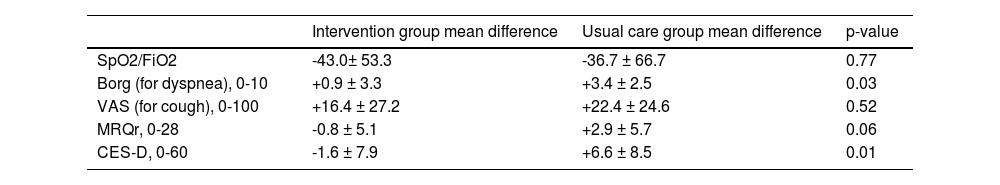
Treatment groups comparisonFocusing on the differences between the values registered for the studied scales between the baseline and after 12 months of treatment, changes in the means of the treatment groups were identified (Table 3). The Borg scale at 12 months showed a worsening in dyspnoea in both groups, such worsening being less evident in the intervention group (+0.93 ± 3.34 vs +3.42 ± 2.5, p-value: 0.03). A similar trend was also observed for the CES-D scale (+1.57 ± 7.95 vs +6.58 ± 8.55, p-value: 0.01). There were no differences for the other scales.
Scales differences between values at the baseline and after 12 months for the two treatment groups. The p-value of the two samples t-test are reported to compare the treatment groups.
Legend. SpO2 = Peripheral oxygen saturation; FiO2 = Fraction of inspired oxygen; VAS = Visual Analogic Scale for cough; MRQr = Maugeri Respiratory Questionnaire reduced form; CES-D = Center for Epidemiologic Studies Depression Score.
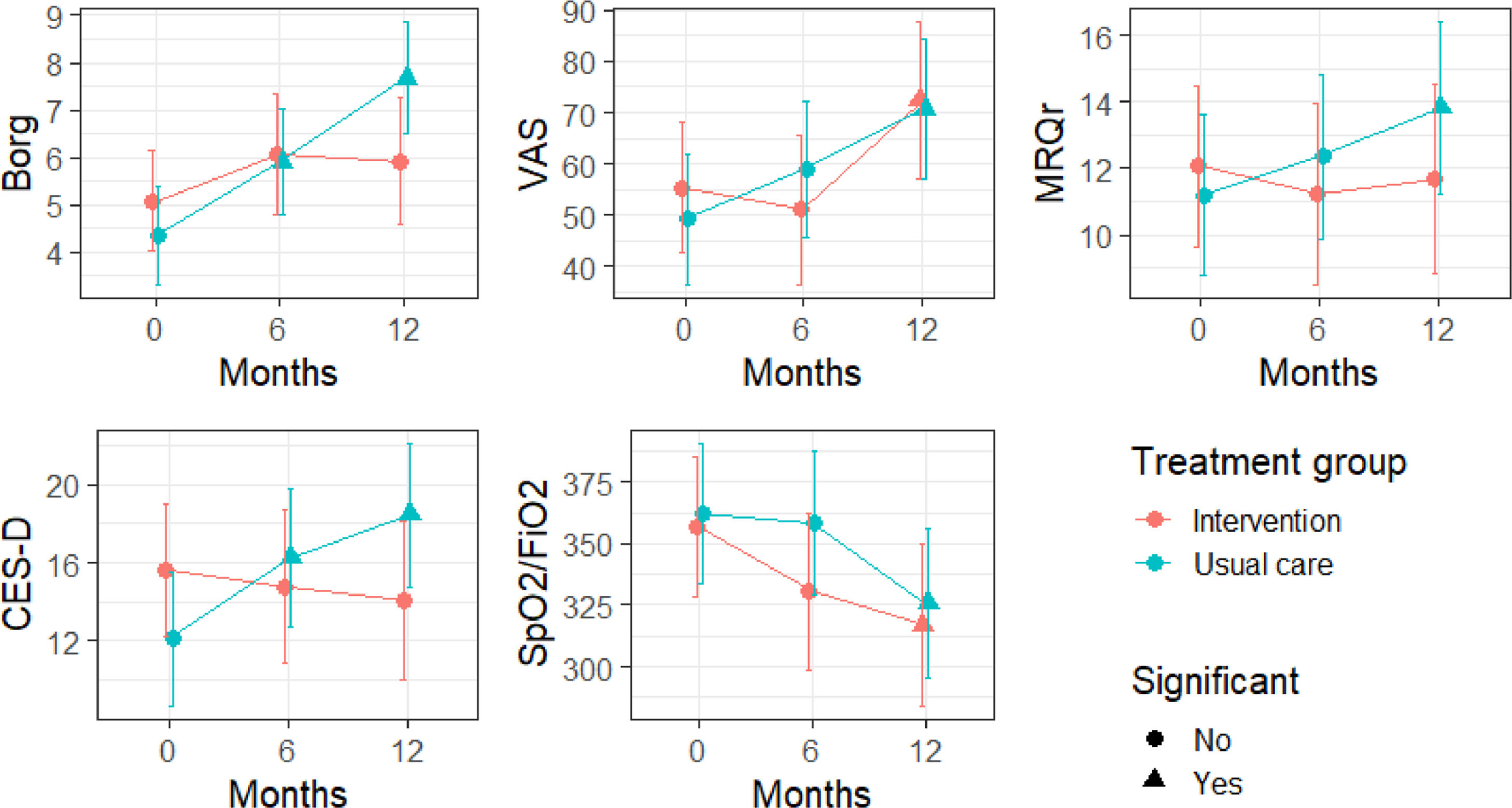
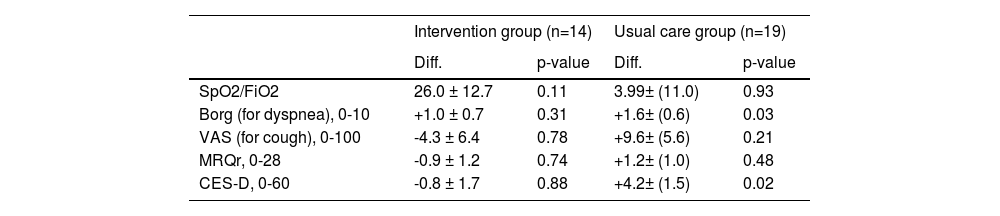
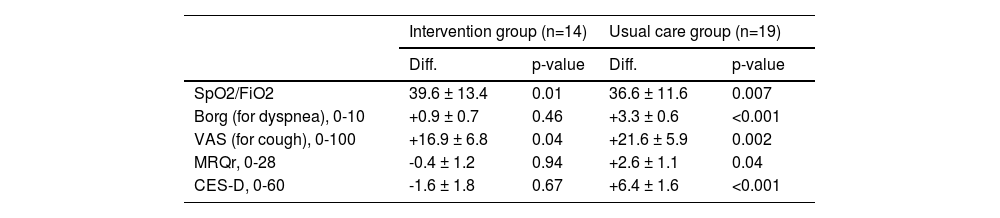
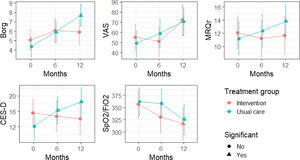
Results concerning the scales differences at 6 month and 12 months are shown in Table 4 and Table 5. Moreover, in Fig. 2, the absolute variations over time for the different scales are shown. We did see an improvement in dyspnoea and depression: specifically, Borg scale's and CES-D scale's values remained stable in patients from the intervention group, while they both deteriorated in patients from the control group. Particularly, changes > 30% in the CES-D represent a minimal clinically important difference.34 In contrast, we noted a constant decline in VAS scale values for cough in both groups: based on such observation, we suppose that palliative care is not sufficient to alleviate the burden of debilitating cough, which most ILD patients share.
Changes after 6 months (n=38). Least squares estimate of the average differences (Diff.) between values observed at the baseline and after 6 months are reported with the correspondent standard error (S.E.) and adjusted p-values for comparisons within each treatment group.
Legend. SpO2 = Peripheral oxygen saturation; FiO2 = Fraction of inspired oxygen; VAS = Visual Analogic Scale for cough; MRQr = Maugeri Respiratory Questionnaire reduced form; CES-D = Center for Epidemiologic Studies Depression Score.
Changes after 12 months (n=33). Least squares estimate of the average differences (Diff.) between values observed at the baseline and after 12 months are reported with the correspondent standard error (S.E.) and adjusted p-values for comparisons within each treatment group.
Legend. SpO2 = Peripheral oxygen saturation; FiO2 = Fraction of inspired oxygen; VAS = Visual Analogic Scale for cough; MRQr = Maugeri Respiratory Questionnaire reduced form; CES-D = Center for Epidemiologic Studies Depression Score.
Fitted values and 95% confidence intervals for the studied scale in time. The triangles highlight a statistically significant difference with respect to the baseline (see Tables 4-5).
Regarding the Borg scale, no differences in time were observed in the intervention group (p-value between baseline and 6 months 0.31, p-value between baseline and 12° month 0.46), while Borg scale values worsened in usual care group (p-value between baseline and 6 months 0.03, p-value between baseline and 12° month < 0.001). Similarly, CES-D scales’ values remained stable in the intervention group's patients (p-value between baseline and 6 months 0.88; p-value between baseline and 12° month 0.67); on the contrary, there was a worsening in CES-D values in usual care group (p-value between baseline and 6 months 0.02; p-value between baseline and 12° month <0.001). The VAS scale values for cough worsened both in intervention and usual care group (p-value group effect: 0.88), especially in the second 6-month period (p-value between baseline and 12° month: 0.04 for the intervention group and p-value 0.002 for the usual care group). A similar pattern can be observed for SpO2/FiO2 (p-value between baseline and 12° month: 0.01 for the intervention group and 0.007 for the usual care group). Regarding the MRQr scale, only a slight decrease in usual care group was detected after 12 months (p-value: 0.04), although no decrease in the intervention group was detected (p-value: 0.94). To test for differences in survival of subjects included in the two groups, Kaplan-Meier curves were estimated: differences were not significant (p-value=0.11).
The mean value of the chair sit-and-reach test at the baseline was 11.1 cm ± 6.6, 10.4 cm ± 10.4 after 6 months, and 10.4 cm ± 8.0 after 12 months. Similarly, lower body strength in the intervention group at baseline, using the 30-s chair stand, was evaluated in all patients, and the mean value was 5.9 ± 3.6 repetitions; 5.2 ± 3.4 repetitions after 6 months and 3.6± 2.8 repetitions after 12 months. Based on a one-way ANOVA model, we did not observe changes in these measures during the study period for either the lower body flexibility or strength.
DiscussionThis randomised pilot study suggests that a comprehensive palliative care program is feasible to implement and can slow the rate of dyspnoea worsening and reduce depression among ILDs patients. To the best of our knowledge, this is one of only a few pilot trials examining the feasibility and efficacy of palliative care – in particular, rehabilitation activities and psychological support – for patients with advanced and fibrosing interstitial lung disease. Notably, 88% of patients were already requiring supplementary oxygen therapy at the baseline, suggesting pulmonary function was severely compromised. Consistent with this observation, the mortality rate was 34% during the 12 months follow-up.
It has been suggested that for patients with chronic lung disease, early palliative care should be started, and therefore, it may be argued that we enrolled patients in a late phase of the disease, where palliative interventions may have limited opportunity for effectiveness. Efficient patient-centred models of palliative care must be validated, considering religious and cultural differences, as well as variability of resources.35 The identification of those most likely to benefit from specialist palliative care seems a crucial point. For example, Kluger et al.36 in a Parkinson's disease palliative care trial, found overall benefit but most benefit in those with high needs.
Including those with low needs may have diminished the impact of the intervention. This may raise an important issue in our study design as the “wait until not-treatable” model of palliative care is a cause of “too little, too late” regarding palliative care service access. Identification of the patients who would benefit by using a needs assessment would be very helpful in designing future studies.37
Patients randomised to the intervention gained an increase in lower body flexibility at 6 months, but at 12 months the median value was similar to that at baseline, as illustrated above. Conversely, at 12 months, lower body strength deteriorated, passing from 5.5 to 3 repetitions. These findings are in line with the expected course of the disease and reinforce the perception that physiotherapy can contribute to improving lower-body flexibility but cannot improve lower body strength in advanced chronic illness. Not surprisingly, palliative care had no impact on patients’ gas exchange, represented by SpO2/FiO2 ratio, which declined in both groups. A Cochrane review on pulmonary rehabilitation demonstrated the safety of this approach and the amelioration in functional exercise capacity, dyspnoea and quality of life, but the considered studies were performed in patients with a different degree of severity. Indeed, because of inadequate reporting of methods and small numbers of included participants, the quality of evidence was low to moderate.38
A recent trial by Janssen et al.14 has explored the impact of palliative care on QoL, depression, and anxiety in patients with IPF. Our results differed from this prior pilot in that the target population was different, and the scales used to evaluate QoL and depression were not the same. Authors suggested that receiving palliative care may cause a worsening symptom-related quality of life in IPF in the short term and a possible transient worsening in depression. The authors note that the study population had mild disease, with an average FVC of 73.4% predicted and a moderate deflection of DLCO (54.9% and 57.8% in the two groups); they concluded that patients receiving palliative care too early in their disease course, and discussions regarding prognosis might have worsened symptoms of depression or anxiety. Their findings were like the study conducted by Lindell et al.,12 in which patients with IPF were receiving care by a clinical nurse specialist, a psychiatric clinical specialist, and an advanced care planning instructor had reduced health related QoL and increased anxiety. However, a recent meta-analysis showed that holistic services for chronic breathlessness can reduce distress in patients with advanced disease and may improve psychological outcomes of anxiety and depression.39 Concerning the effects on dyspnoea, we have confirmed the data collected by Higginson et al.40 that showed how the breathlessness support service improved breathlessness mastery in patients with diseases other than cancer, but with very few patients with ILD.
Sample size calculation for further multicenter RCTsIntervention group did better than usual care group for at least one of the primary outcomes (reducing the increase of dyspnoea during the time course). We may suggest therefore that this variable should be considered in designing a phase III study. A 6 months study will allow us to detect some benefits quicker, and eventually reduce the loss of number of patients due to their deaths. Indeed, when we consider feasibility for an outpatients based-program, we also have to take into consideration other items such as costs of transportations, loss of working-days by caregivers, and distance from the hospitals. We estimate that, considering CES-D and Borg scale as primary outcomes at 6 months and assuming an equal enrolment rate by centers, a sample size of 146 patients (total of intervention + control), is required to obtain a power of 0.80, fixing the intra-center correlation equal to 0.1.
LimitationsOur pilot trial has several limitations. First, this is a single-center study and may not generalize to other areas; indeed, very few (if any) of the Respiratory Units in our Country have a structured palliative care program for ILDs patients, so that even psychological support is not usually provided, if not as inpatients. This may be different in other geographical locations.12,40 Second, we had a relatively small sample size: a larger randomised trial is necessary to confirm our findings. Third, the chair stand and chair sit and reach tests were not recorded in the usual care group, and it was not possible to record pulmonary function tests (PFTs) in most of patients due to severe impairment and subsequent difficulties in performing them, especially concerning DLCO, most of them being on oxygen therapy. In keeping with the last point, some patients could not perform the six minute walking test (6MWT) and, most important, a portion of those who could perform the test (14/25) were not able to repeat it at 12 months, due to the pandemic, since the dedicated corridor was closed to outpatients. Lastly, the exclusion of patients who were not taking anti-fibrotic medication, limit the generalization of our results in patients receiving these drugs.
ConclusionsOur study demonstrated the feasibility and the efficacy of multi-disciplinary palliative care intervention including counseling, pulmonary rehabilitation and psychological support in patients with advanced ILD. This approach was associated with evidence of less and slower worsening during one year observation period, in dyspnoea and depression compared to those patients receiving usual care. Additional studies are needed to confirm the efficacy and identify those patients who most likely can benefit from different intervention components and the best timing for implementation.
All authors contributed equally to the work.
Stefano Nava has been identified as the guarantor of the paper, taking responsibility for the integrity of the work, from inception to published article.
This work was supported by the no-profit organization “Re-use-with-love”, Bologna, Italy.
This manuscript is dedicated to the memory of Giorgio Gambale, M