Several multidimensional indices have been proposed to predict mortality in chronic obstructive pulmonary disease (COPD). The BODEX index is simple and easy to use for this purpose in all clinical settings. Only a few prognostic indices have integrated oxygenation variables, with measurement methods that are not practical for real life clinical practice in all settings.
ObjectivesTo develop and externally validate a new prognostic index (BODEXS90) that combines the variables included in BODEX index with rest peripheral oxygen saturation measured with finger oximetry (SpO2) to predict all-cause mortality in stable COPD.
MethodObservational, non-intervention, multicenter historic cohort study. The BODEXS90 index was developed in a derivation cohort and externally validated in a validation cohort. Calibration of the index was carried out using Hosmer-Lemeshow test. The discrimination capacity of BODEXS90 and BODEX were compared by means of receiver-operating characteristics curves. Modelling of the index was carried out by crude and adjusted Cox regression analysis.
ResultsThe derivation and validation cohorts included 787 and 1179 subjects, respectively. SpO2 predicted all cause-mortality independently of BODEX index. Discrimination capacity of BODEXS90 to predict the outcome was significantly higher than that of BODEX, particularly for more severely affected patients, both in the derivation and in the validation cohorts.
ConclusionsThe new index is potentially useful for designing clinical decision-making algorithms in stable COPD.
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of death in the world.1 Therefore, predicting mortality is of paramount importance to design management strategies for this disease. Traditionally, the forced expiratory volume in one second (FEV1) has been the most widely used predictor of mortality. However, it is currently clear that COPD is a complex disease, with multiple dimensions involved in its prognosis. As a consequence, several multidimensional indices have been validated, integrating assorted prognostic determinants, with the objective of improving the predictive capability of adverse outcomes provided by FEV1 alone.2 In this regard, the BODE (body mass index, airflow obstruction, dyspnea, exercise capacity) index is more effective than FEV1 in predicting mortality.3 However, the index does require performance of a 6-minute walk test, a technique that is time-consuming and requires sufficient space to perform it, making it difficult to be carried out in some settings, like primary care facilities. The BODEX index simplifies the BODE index and precludes these drawbacks by replacing the exercise capacity component with the history of COPD exacerbations that required hospitalization the year prior to patient...s evaluation.4 This index has a similar prognostic capacity to BODE.4
Chronic respiratory failure is associated with a higher mortality rate in COPD, and it is another dimension that is amenable to treatment, with long-term oxygen therapy.5 For that reason, oxygenation variables might be theoretically valuable for use as a component of prognostic indices in COPD. Two previously validated indices, mBODE and DOREMI BOX have integrated these variables, but they used measurement methods (maximal oxygen consumption during exercise test and blood gas analysis, respectively) that are difficult to implement in all clinical settings and throughout the whole spectrum of disease severity.6,7 On the other hand, measurement of peripheral oxygen saturation by means of pulse oximetry (SpO2) correlates well with arterial partial pressure of oxygen,8 it is cheap, easy and readily available in all clinical scenarios.
We hypothesized that a new index that combines rest SpO2 with the BODEX index variables would increase the capacity of BODEX index to predict all-cause mortality in stable COPD patients. The objectives of this study were: 1) to determine whether rest SpO2 and BODEX index are independent predictors for death, 2) to develop an index that combines the BODEX components with SpO2 (BODEXS90), 3) to assess whether the prognostic capacity of BODEXS90 is higher than that of BODEX in a derivation cohort and 4) to externally validate the index in an independent cohort.
MethodsStudy population and settingThis was an observational, non-intervention, multicenter historic cohort study. Inclusion criteria were age > 35 years, a diagnosis of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD)1 and clinical stability at the time of the first visit (i.e.: free of exacerbations in the prior 3 months). Exclusion criteria were the diagnosis of concomitant chronic respiratory diseases other than COPD (e.g: interstitial lung disease, pneumoconiosis). The derivation cohort contained consecutive patients seen at a second-level university hospital...s dedicated COPD clinic (Hospital Universitario Lucus Augusti, Lugo, Spain), visited from January 2008 to October 2019. The participants were identified from a database that was set-up for clinical purposes. The validation cohort was made up of a combination of three different cohorts: two were prospective observational cohorts from third-level University hospitals (Hospital de Galdakao-Usansolo, Galdakao, Spain and Hospital Universitario Marqu..s de Valdecilla, Santander, Spain) recruited from April 2003 to November 2004 and from March 2018 to August 2018, respectively. The third one was a clinical cohort, formed of all consecutive patients seen at a general pulmonology clinic from a 3rd level university hospital (Hospital Universitario Nuestra Se..ora de Candelaria, Santa Cruz de Tenerife, Spain) which was visited from January 2012 to December 2014.
Study variablesOn the first visit, the following variables were registered: age, sex, history of tobacco consumption (pack-year index, current versus former smoker), body mass index (kg/m2), value of the age-adjusted Charslon comorbidity index,9 percent-predicted forced expiratory volume in 1-second (FEV1%), percent-predicted forced vital capacity (FVC%), FEV1/FVC index, value of SpO2 measured with the patient resting in sitting position, dyspnea measured using the modified medical research council scale (mMRC), and number of exacerbations that required hospital management during the year previous to the first visit. The BODEX index was calculated as previously described.4 Introducing SpO2 in a multivariable Cox proportional hazard regression analysis did not significantly change the hazard ratios (HR) of the BODEX variables to reach the endpoint outcome (see below), and the HR associated to a SpO2 below 90% was 1.6. Thus, the new BODEXS90 index was computed by adding one point to the BODEX index when SpO2 was below 90% (Table 1).
Variables and score values used for the calculation of the BODEXS90 index.
| Score | |||||
|---|---|---|---|---|---|
| Variable | 0 | 1 | 2 | 3 | |
| B | BMI (Kg/m2) | > 21 | ..± 21 | ||
| O | FEV1% | ... 65 | 64 ... 50 | 49 -36 | ..± 35 |
| D | MMRC dyspnea scale | 0 ... 1 | 2 | 3 | 4 |
| EX | Number of COPD exacerbationsa | 0 | 1 -2 | ... 3 | |
| S90 | Resting SpO2 | ... 90% | < 90% |
All-cause mortality was the outcome variable of the study. Vital status was determined by reviewing electronic medical records and, in some cases, by telephone calls to patients or relatives. Dates of death were obtained from the medical records.
Statistical analysisDescriptive analyses were carried out by calculating mean and standard deviation for continuous variables and frequency and percentages for discrete variables. Comparisons between the derivation and validation cohorts were performed by means of Student...s T-test or Pearson...s chi-square test, as appropriate. To assess whether BODEX and SpO2 had independent value as predictors of mortality, a Cox proportional hazards regression analysis was carried out in the derivation cohort, by introducing both variables simultaneously. Both BODEX index and SpO2 were coded continuously, in one-unit increments. The ability of BODEX and BODEXS90 indices to predict mortality was compared by means of receiver-operating characteristics (ROC) curves. For this analysis, the indices were coded continuously, in 1-unit increments. We included a ROC analysis for the most commonly used predictor variable (FEV1%), as a comparator. The areas under the curves were compared using the method of De Long et al.10
Calibration of the index was performed by fitting a multivariate logistic regression model and using Hosmer-Lemeshow goodness-of-fit test. All-cause death was the dependent variable and predictors were BODEXS90 index plus age-adjusted Charlson index, pack-years index, and current smoking status. BODEXS90, Charlson and pack-years indices were coded continuously, in 1-unit increments, while current smoking status was coded dichotomously (yes/no).
We used Cox proportional hazards regression models to calculate HR for mortality and 95% confidence intervals (CI) for BODEX and BODEXS90. We calculated crude (model 1) and adjusted (model 2) HR. In model 2, we adjusted for pack-years index, current smoking status and age-adjusted Charlson index, coded as previously mentioned. To enhance the applicability of the indices for clinical practice, BODEX and BODEXS90 were divided into quartiles for this analysis3,4 (Table 2).
Survival curves for BODEXS90 index, divided into quartiles, were constructed using the Kaplan-Meier Method. The curves were compared by means of the log-rank test.
All the analyses were repeated in the validation cohort using the same methodology. All effects were considered significant at a p-value < 0.05. The statistical analysis was performed with MedCalc statistical software Version 13.3.3.0 (MedCalc Software bvba, Ostend, Belgium)
We did not use an a priori sample size calculation because this is a retrospective analysis of cohorts that recruited patients for other studies. Further, there are no overall accepted methods to estimate the sample size for derivation and validation studies of risk prediction models.11 It has been suggested that an adequate sample size for these studies should include a number of participants ... 20 with the outcome event per candidate variables for derivation cohorts, and a number of at least 100 events for validation cohorts.12 Our sample and the number of events exceeded these thresholds (see results).
Compliance with ethical standardsThe collection of clinical data from the medical records was originally authorized by the ethical committees. We obtained specific authorization to carry out the present study (Comit.. de ..tica de Investigaci..n Cl.ínica del Hospital Universitario Nuestra Se..ora de Candelaria, Registry number: CHUNSC_2020_52). The data were de-identified for analysis. Informed consent was waived for this analysis due to the retrospective, non-interventional design of the study and the use of anonymous clinical data for the analysis.
ResultsThe derivation cohort included 792 patients and the validation cohort included 1234 subjects. Five patients were lost to follow-up in the derivation cohort and 55 in the validation cohort, mainly because the patients moved to other areas or because they had withdrawn from the original prospective studies. Thus, the final sample size for the derivation and the validation cohorts were 787 and 1179, respectively. As contemplated by the Spanish COPD guidelines,13 all the study variables are systematically registered in the participant centers, thus there were few missing data. Only 15 (1.9%) cases in the derivation cohort and 32 (2.7%) subjects in the validation cohort had missing data. Therefore, since the risk of bias was low, imputation techniques were not deemed necessary and complete case analysis was carried out.14
Table 3 shows the characteristics of the derivation and validation cohorts. There were significant differences in most variables and, in general, the patients from the validation cohort had less severe disease. Follow-up time was similar for both cohorts.
Characteristics of the derivation and validation cohorts.
| Derivation cohort (N...=...787) | Validation cohort (N...=...1179) | P | |
|---|---|---|---|
| Age, years | 67.8........9.5 | 68.0........8.8 | 0.63 |
| Male sex, n (%) | 697 (88.5) | 826 (70.0) | < 0.0001 |
| Packs-year | 58.6........30.0 | 49.1........24.4 | < 0.0001 |
| Current smokers, n (%) | 212 (26.9) | 139 (11.7) | < 0.0001 |
| SpO2, % | 93.1........4.4 | 94.3........2.7 | < 0.0001 |
| Subjects with SpO2...<...90%, n (%) | 128 (16.2) | 66 (5.5) | < 0.0001 |
| FEV1, % | 50.4........17.2 | 55.5........17.2 | < 0.0001 |
| FVC, % | 74.8........17.5 | 81.9........19.5 | < 0.0001 |
| FEV1/FVC, % | 48.8........12.5 | 52.4........11.2 | < 0.0001 |
| GOLD 1, n (%) | 44 (5.5) | 88 (7.4) | 0.11 |
| GOLD 2, n (%) | 351 (44.5) | 643 (54.5) | < 0.0001 |
| GOLD 3, n (%) | 283 (35.9) | 379 (32.1) | 0.08 |
| GOLD 4, n (%) | 109 (13.8) | 69 (5.8) | < 0.0001 |
| BMI, kg/m2 | 28.2........5.9 | 28.1........4.9 | 0.68 |
| BODEX index | 2.5........1.9 | 2.5........1.7 | 1.00 |
| Charlson indexa | 5.3........1.9 | 4.9........2.0 | < 0.0001 |
| Follow-up time, months | 56.0........30.1 | 56.3........32.5 | 0.83 |
| Deaths, n (%) | 217 (27.5) | 321 (27.2) | 0.92 |
Age-adjusted. FVC: forced vital capacity. GOLD: global initiative for chronic obstructive lung disease. For further definitions see legend to Table 1.
Table 4 shows the results of the Cox proportional hazards analysis to assess whether BODEX and SpO2 had independent value as predictors of mortality. Both variables independently predicted the risk for all-cause death both in the derivation and the validation cohorts.
Results of the Cox proportional hazards regression model to assess the independent value of BODEX and SpO2 to predict mortality in the derivation and validation cohorts.
| Derivation cohort | Validation cohort | |||
|---|---|---|---|---|
| Covariate | HR (95% CI) | P | HR (95% CI) | P |
| BODEX | 1.28 (1.19...1.38) | < 0.0001 | 1.23 (1.15...1.32) | < 0.0001 |
| SpO2 | 0.93 (0.91 ... 0.96) | < 0.0001 | 0.93 (0.90 ... 0.97) | 0.0009 |
HR: hazard ratio. CI: confidence interval. For further definitions see legend to Table 1.
Area under the ROC curve (AUC) to predict mortality for BODEXS90 in the derivation cohort was 0.753 (95% CI: 0.722 ... 0.783), slightly but significantly higher than the AUC for BODEX: 0.745 (95% CI: 0.713 ... 0.775), difference between AUC: 0.008 (95% CI: 0.002 ... 0.013), p...=...0.006, and higher than the AUC for FEV1%: 0.687 (95% CI: 0.654 ... 0.720), difference: 0.065 (95% CI: 0.037 ... 0.094), p...<...0.001.
The AUC for BODEXS90 and BODEX in the validation cohort were 0.670 (95% CI: 0.640 ... 0.695) and 0.663 (95% CI: 0.636 ... 0.691), respectively. The difference between AUC was small but also statistically significant: 0.007 (95% CI: 0.0001 ... 0.008), p...=...0.04. The AUC for BODEXS90 was also higher than that of FEV1% in this cohort: 0.617 (95%: 0.588 ... 0.645), difference: 0.051 (95% CI: 0.026 ... 0.076), p...<...0.001.
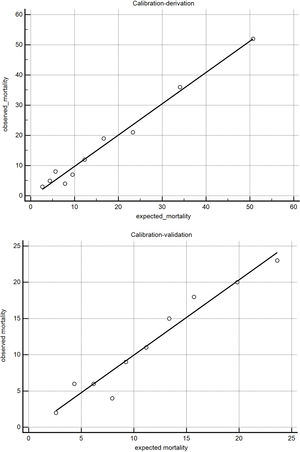
The BODEXS90 provided an adequate match between predicted and observed mortality (Fig. 1). The value of the Hosmer-Lemeshow statistic was 5.21 in the derivation cohort and 12.8 in the validation cohort (p...=...0.73 and 0.11 with 8 degrees of freedom, respectively).
The HR for mortality for the highest quartiles of BODEXS90 were higher than the highest quartiles of BODEX, both in crude and adjusted models, and in the derivation and validation cohorts, suggesting that BODEXS90 is a better predictor of mortality than BODEX (Table 5).
Hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality, for BODEX and BODEXS90 indices coded in quartiles, in derivation and validation cohorts. Model 1: crude HR. Model 2: HR adjusted for pack-year index, current smoking status and age-adjusted Charlson index.
| Derivation cohort | ||||
|---|---|---|---|---|
| BODEX | BODEXS90 | |||
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | 3.07 (2.19...4.31) | 2.84 (1.93...4.16) | 2.86 (2.00...4.08) | 2.57 (1.72...3.86) |
| Q3 | 4.44 (3.05...6.46) | 4.24 (2.79...6.45) | 4.95 (3.47...7.06) | 4.95 (3.32...7.37) |
| Q4 | 10.02 (6.22...16.16) | 8.02 (4.34...14.79) | 11.6 (7.07...19.11) | 8.48 (4.42...16.24) |
| Validation cohort | ||||
|---|---|---|---|---|
| Q1 | Reference | Reference | Reference | Reference |
| Q2 | 1.74 (1.35...2.24) | 1.63 (1.25...2.12) | 1.68 (1.30...2.17) | 1.59 (1.22...2.08) |
| Q3 | 2.61 (1.95...3.50) | 2.38 (1.75...3.24) | 2.65 (2.00...3.50) | 2.38 (1.78...3.19) |
| Q4 | 4.72 (2.61...8.55) | 3.88 (2.14...7.05) | 7.29 (3.21...16.53) | 6.69 (2.93...15.29) |
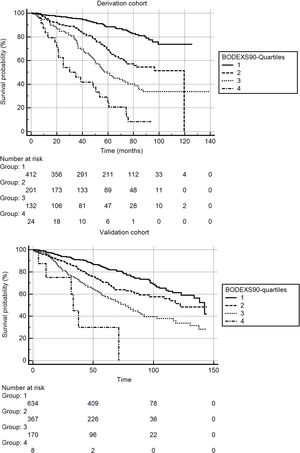
Fig. 2 shows the Kaplan-Meier survival curves for the BODEXS90 quartiles in the derivation and validation cohorts. The differences between curves were significant for both cohorts (p...<...0.0001 for both samples).
DiscussionThe present study has shown that SpO2 has additional prognostic value to predict mortality in stable COPD patients, independent of BODEX index, and that combining oximetry results with the BODEX index components, in a new composite BODEXS90 index, increases the ability to predict all-cause mortality in this population, particularly in the most severe cases.
COPD is a major health problem worldwide. Predicting mortality in this disease is an important element to design follow-up and treatment strategies. COPD is a heterogenous, multi-component disease, and using a single dimension, like lung function variables, to predict mortality does not take into account the complexity of the factors that can influence the prognosis of patients. The GOLD initiative acknowledges the value of composite scores to predict disease outcomes and recognizes that the BODE composite score is a better predictor of survival than individual components of the index.1 GOLD also admits that simpler composite indices that do not include exercise test might be suitable alternatives. These indices might be easier to use in non-specialized settings, but validation studies are needed before they can be used in clinical practice.1
Many prognostic models have been studied in COPD, although only a minority has been externally validated.15 Some of these, like the ADO index include non-modifiable variables, like age, that are not directly related to the disease in itself.11,16 The value of age and similar, non-modifiable variables in the clinical stratification of specific diseases has been questioned.17 Although such variables, integrated into multidimensional indices are useful to inform patients and relatives about prognosis, and to help regulatory agencies to manage health resources, their utility to tailor individual treatments is less evident. On the other hand, prognostic indices integrated by components that are susceptible to modification with treatments should be more useful when designing therapeutic strategies. They could be used to measure the impact of interventions and, ideally, the improvement of index scores with treatments should correlate with better clinical outcomes. Actually, the BODE index has been found to improve with therapeutic interventions, like pulmonary rehabilitation, and to correlate in this context with better outcomes.18 However, general implementation of BODE index is hampered by the need to carry out an exercise test. Also, patients with some comorbidities, like orthopedic or peripheral vascular diseases might not be able to perform the test, limiting its widespread availability. The simpler BODEX index, which does not require a 6-minute walk test, and replaces it by the history of severe exacerbations is advantageous in this regard.4 This index has been externally validated by indepent teams since its development, and the value of the c-statistic to predict adverse outcomes for this model was between 0.63 and 0.73 in a recent meta-analysis.15
Low SpO2 identifies COPD cases with more severe disease, and correlates with poorer survival.19 Long-term oxygen therapy is one of the few interventions that have demonstrated an impact on survival of COPD patients with respiratory failure. Hence, oxygenation variables are attractive candidates for becoming part of prognostic indices, particularly in those aimed to measure the impact of therapeutic interventions. A previous paper evaluated the ability of a modified BODE (mBODE) index, that used oxygen uptake measured at peak exercise during cardiopulmonary exercise test, to predict mortality in COPD.6 Surprisingly, the conventional BODE index performed equally well, if not even slightly better than mBODE to predict mortality. The authors speculated that some patients might have stopped the exercise test because of dyspnea or leg fatigue before reaching a true peak oxygen uptake, and concluded that simpler tests might be more practical to evaluate the multidimensional deterioration of COPD patients.6 Our proposed BODEXS90 index is simple and easy to obtain. Global discrimination of the index, assessed with the c-statistic values, was only marginally higher than that of BODEX, and of uncertain clinical significance.20 However, modeling of the index showed that it might be more useful than BODEX to predict mortality, particularly for those patients at higher risk (i.e: those in the highest quartiles of both indices). Therefore, the index might be particularly helpful for outcome prediction in more severe cases. It must be noted that BODEXS90 conserved its predictive value after adjusting for two important confounders, age and comorbidity, quantified with the age-adjusted Charlson index. There are two possible reasons that might explain the small increase of the c-statistic values of BODEXS90 over BODEX: the number of patients with SpO2 < 90% was low, particularly in the validation cohort. Thus, the study might lack power to adequately assess the discrimination ability of the index. Second, it is plausible that most patients with low oxygen saturation might have been treated with supplementary oxygen, and this might have reduced the impact of this variable on the study outcome. Due to the design of the study, this possibility cannot be reliably ruled out.
The new index proposed in this study is potentially useful for future research. Comorbidities are common in COPD patients21 and they can have an adverse effect on survival in this disease. Combining COPD-specific prognostic indices (e.g: BODEX or BODE) with comorbidity indices (e.g: Charlson or COTE) into new, combined indices, increases the ability to predict all-cause mortality.22,23 Therefore, combining BODEXS90 with a comorbidity index might also prove advantageous in this respect. Also, COPD is a complex disease24 and it has been found that mortality risk is different for distinct clinical phenotypes.25 It is plausible that patients with emphysema are at a higher risk of suffering oxygen desaturation than subjects with other phenotypes. Thus, the performance of BODEXS90 may vary based on patients... phenotype.
The present study has some strengths: it incorporated a relatively high number of patients with a long follow-up, and it included an external validation cohort. Because the study variables are systematically registered by the investigators in their clinical practice, missing values were few and the risk of bias is low. The outcome variable (all-cause mortality) is robust and easy to measure in our public health system, which covers virtually the whole population, and uses electronic medical records. There were significant differences in the characteristics of patients in the derivation and validation cohorts. This can be considered a strength of the study, because it is recommended that validation studies should be performed in populations with a different case mix than the derivation cohorts.26 Conversely, some limitations must be acknowledged: the study was performed in pneumology services, and the number of patients with less severe ventilatory obstruction (i.e. GOLD-1) was low. This is a significant limitation, particularly for an index that purports to be useful in all clinical settings. Additional studies should be carried out including patients followed-up in a primary care setting. As mentioned previously, the number of patients with SpO2 < 90% was low, and the sample size might not be large enough to adequately assess the differences in the discriminative ability of BODEXS90 and BODEX indices. There were even fewer patients with SpO2 < 85% and, as a consequence, we could not evaluate whether using other cut-off values for SpO2 might increase the discrimination capability of BODEXS90. Besides, the outcome variable was measured over a long follow-up period. If the index is to be useful for planning ahead therapeutic strategies, its ability to predict outcomes should be ideally demonstrated within a shorter time frame (i.e: 12 months), and a larger sample size would be needed to demonstrate this.
Despite these limitations, the study shows that SpO2 has additional prognostic value over the previously validated multi-component BODEX index and that a simple, easy to obtain multidimensional BODEXS90 index that includes oxygenation variables can improve the ability of the former to predict long-term all-cause mortality. These results provide the basis for future validation studies from independent investigators teams and to design impact studies to evaluate the effect of using such index in direct decision-making algorithms.
Declaration of conflicts of interestNone.