Deciduoid mesothelioma is a rare variant of epithelioid mesothelioma; it was initially thought that it only occurred in the peritoneum of young women and had nothing to do with asbestos exposure. However, since these early findings it has also been observed in the pleura and the pericardium, with possible association to asbestos. In general the prognosis is poor compared to epithelioid mesothelioma. 45 cases have been reported in the literature up to now, 22 of these were located in the pleural cavity. The authors describe a case of deciduoid pleural mesothelioma in a 40-year-old-woman who presented with right pleuritic chest pain, with no history of asbestos exposure, treated with chemotherapy followed by surgery and who died postoperatively.
O Mesotelioma Deciduóide é um subtipo raro de mesotelioma epitelióide, inicialmente encontrado apenas no peritoneu de mulheres jovens, sem relação com exposição a asbestos. No entanto, desde as primeiras descrições tem sido igualmente diagnosticado na pleura e no pericárdio e pode estar associado aos asbestos. De um modo geral tem pior prognóstico quando comparado com o mesotelioma epitelióide. Há apenas 45 casos descritos na literatura até à data, 22 destes localizados na cavidade pleural. Os autores descrevem um caso de mesotelioma deciduóide pleural numa mulher de 40 anos com toracalgia pleurítica direita, sem história de exposição aos asbestos, submetida a quimioterapia seguida de ressecção cirúrgica, que veio a falecer no pós-operatório.
Malignant mesothelioma is the most common primary malignant tumor of the pleura and the incidence is increasing in the United States, Europe, Japan and Australia. There are four major histological subtypes – epithelioid, sarcomatoid, desmoplastic and biphasic.
Deciduoid mesothelioma, a rare variant of epithelioid mesothelioma, was first described by Talerman et al.1 in 1985 and Nascimento et al.2 in 1994, in the peritoneum of young women and was considered initially to be restricted to this group of patients. However since then there have been cases described showing this kind of tumor in men and older women and also located in the pleura and pericardium. Although initially it was thought that there was no connection with asbestos exposure, several cases described in the literature have been asbestos related. The first reports of deciduoid pleural mesothelioma came out in 20003 and to date 22 cases of this tumor in the pleural cavity have been described.4
The authors describe a clinical case of deciduoid pleural mesothelioma in a 40-year-old-woman with no history of asbestos exposure.
Case reportA previously healthy 40-year-old-woman, who was a non-smoker, complained of right pleuritic chest pain over the previous month and a half, with no other symptoms. She was prescribed analgesic and antibiotic therapy which gave no relief. A chest X-ray showed a homogeneous hypo-transparency in the lower third of the right hemithorax and occlusion of the costophrenic angle consistent with pleural effusion which motivated the General Practitioner to send her to the Emergency Room of Coimbra University Hospital. Her medical background included a cesarean delivery 2 weeks before the onset of chest pain, no drug habits and no history of occupational hazard or asbestos exposure (she worked as a secretary in a Public Department). Also, there was no family history of malignancy.
On physical examination, her temperature was 38°C, she had no respiratory distress and her lung examination showed decreased breath sounds over the lower third of the right hemithorax, dullness on percussion and decreased transmission of vocal vibrations.
Arterial blood gases analysis showed pH 7.45; PaO2 78mmHg; PaCO2 37mmHg; SatO2 96%. Laboratory tests revealed normochromic normocytic anemia (hemoglobin 11.9g/dL; reference value 12–15g/dL), thrombocytosis (742×109platelets/L; reference value 150–400×109platelets/L), leukocytosis (10.9×109cells/L; reference value 4–10×109cells/L), altered coagulation, elevated C-reactive protein (19.77mg/dL; reference value 0–0.5mg/dL) and liver enzymes. The Chest X-ray performed in the Emergency Room showed radiological worsening and progression of the pleural effusion, which occupied the lower third of the right hemithorax.
The patient was admitted to the Pulmonology Department. A diagnostic thoracentesis was performed and on the 5th hospital day a chest drain was inserted in the right pleural cavity due to progression of the pleural effusion, occupying more than one-half of the right hemithorax. Macroscopically the pleural fluid was serous fluid and further analysis revealed a predominance of neutrophils (44%) with 38% lymphocytes, negative culture, a pH of 7.71, low adenosine desaminase (15.5U/L), high LDH (1359U/L) and negative cytology for tumor cells. A total of 2500cc of pleural fluid was drained over 9 days.
Blood cultures and urinary antigen tests for Streptococcus pneumonia and Legionella pneumophila serogroup 1 were negative. Tumor markers CYFRA 21.1, SCC, CA 125 and CA 15.3 were elevated.
Chest computed tomography (CT) revealed parenchymal inflammatory densification areas with air-bronchogram in the right upper lobe, middle lobe and left lower lobe, right hilar adenopathies, right organized pleural effusion and a nodular lesion with 5.8cm×3.3cm in the right costophrenic sulcus invading the 8th rib and thoracic wall with pleural origin.
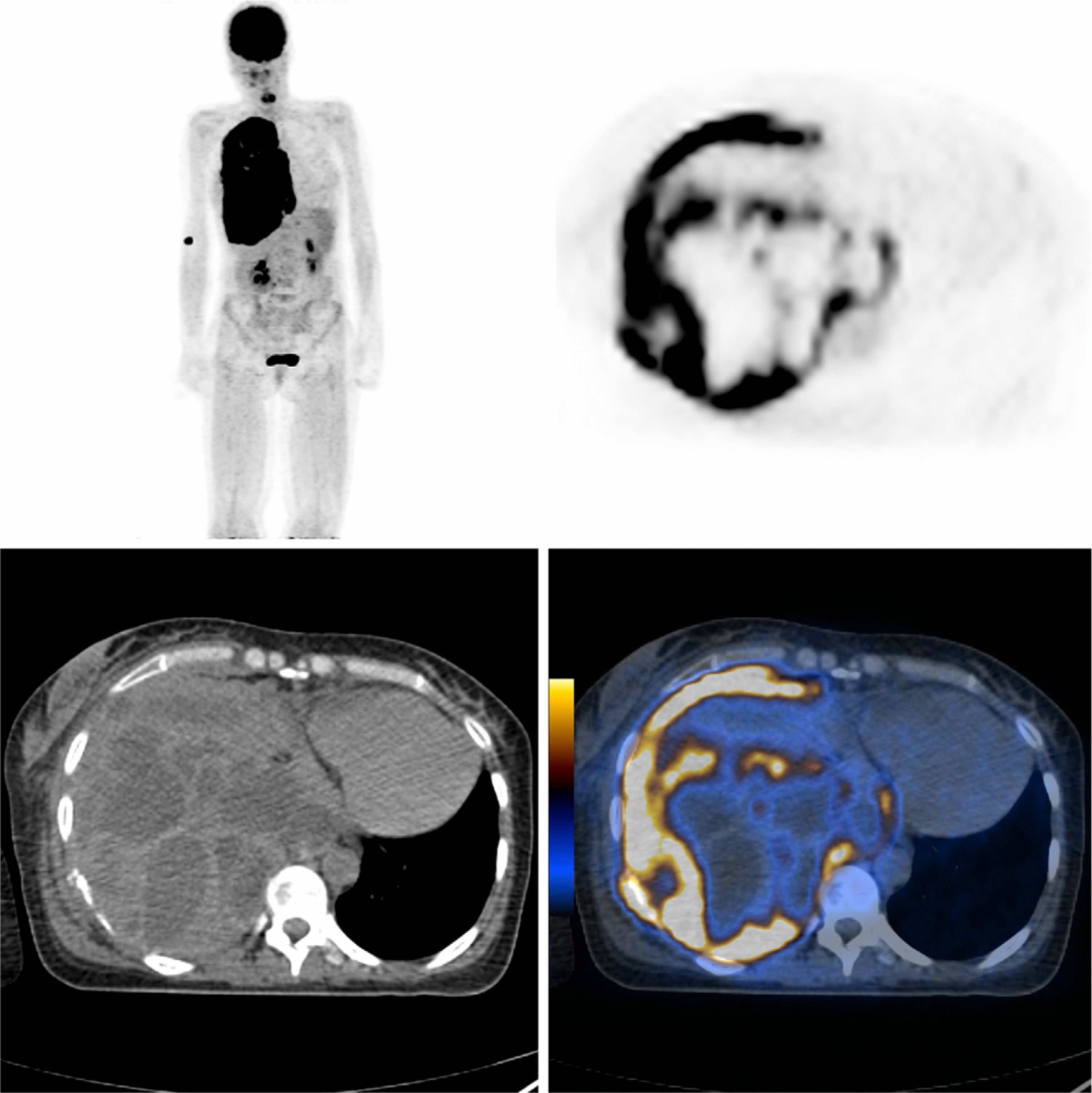
Fiberoptic bronchoscopy showed no endobronchial changes. Tracheo-bronchial aspirate cultures were negative. Abdominal ultrasound was also normal. Positron emission tomography/computed tomography (PET/CT) demonstrated an extensive right pleural hypermetabolic involvement with high probability of malignancy, a mass in the postero-inferior area which invaded the adjacent thoracic wall (mean and maximum standardized uptake value – SUV – 15 and 22, respectively), a suspicious homolateral pleural effusion and secondary mediastinal and bronchohilar lymph node involvement (Fig. 1).
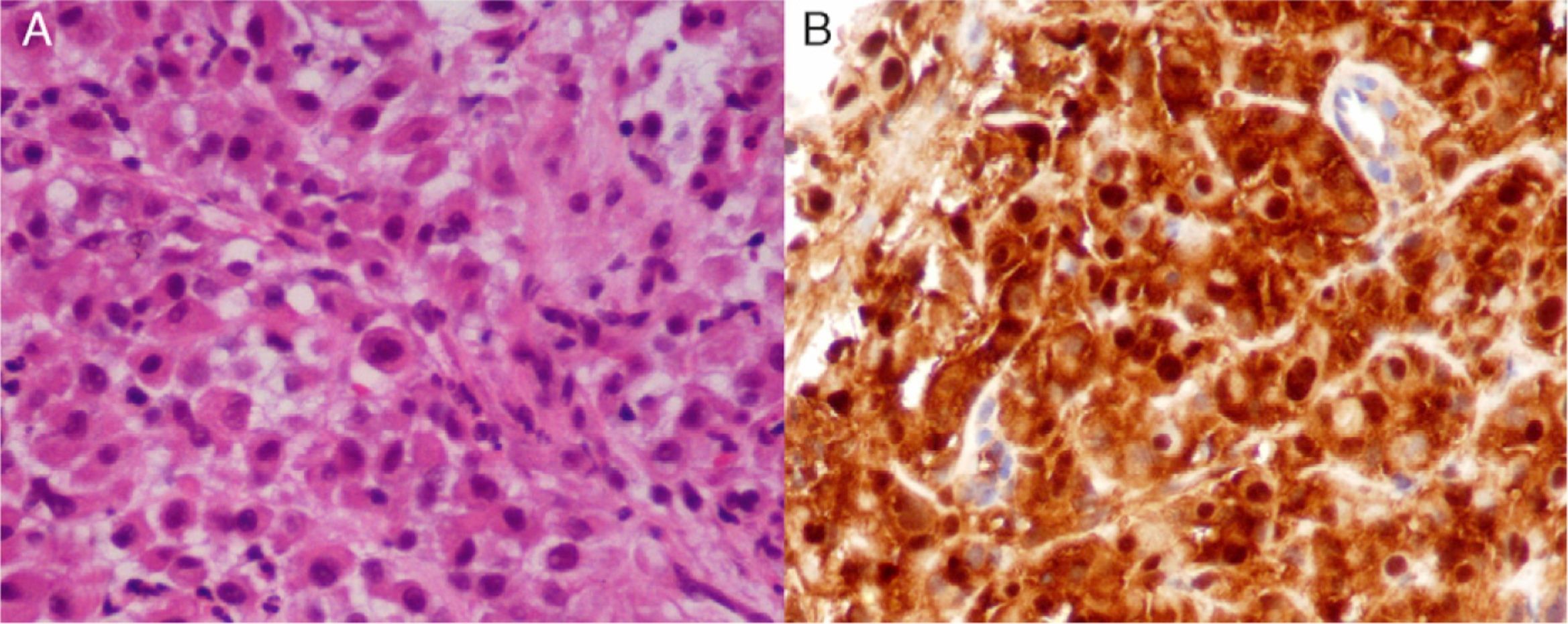
Transthoracic pleural mass biopsy was performed. Light microscopic findings showed malignant discohesive large epithelioid cells with eosinophilic cytoplasm and large round nuclei (Fig. 2A). These cells showed cytokeratin 7 cytoplasmic positivity as well as nuclear and cytoplasmic positivity for calretinin (Fig. 2B) and nuclear p53 positivity. Therefore, deciduoid pleural mesothelioma was diagnosed. Staging according to International Mesothelioma Interest Group (IMIG)5 was T3 N2 M0 (Stage III).
Chemotherapy was started with cisplatin and pemetrexed. After 3 cycles of chemotherapy, reevaluation PET/CT showed progressive chest wall involvement but no extrathoracic metastasis. The patient was accepted for radical surgery, which included right extra pleural pneumonectomy (EPP) with resection of the 5th, 7th and 8th ribs. In the surgical specimen the diagnosis was confirmed morphologically after observation of multiple sections of an extensive lung-encasing yellowish tumor, maintaining the pattern previously observed. The patient died postoperatively, on the second day, of multi-organ failure, 3 months after the initial diagnosis.
DiscussionMalignant deciduoid mesothelioma is a very rare variant of epithelioid mesothelioma, accounting for less than 5% of mesotheliomas6 and according to another author for less than 2%.7 Talerman et al.1 in 1985 were the first to describe the deciduoid aspects of malignant mesothelioma in the peritoneum of a 13-year-old girl. In 1994 Nascimento et al.2 reported a case of a peritoneal lesion in a young woman with morphological features similar to Talerman's description and named it deciduoid mesothelioma. Based on early descriptions, this variant was considered to be restricted to the peritoneum of young women with no history of asbestos exposure. However, later reports have also shown its presence in the pleura and pericardium in elderly people, both female and male, and sometimes in association with asbestos exposure. In the case described the patient had no history of asbestos exposure.
Some cases of malignant deciduoid mesothelioma, in the pleura and peritoneum, diagnosed during pregnancy have been reported,2,8 and a case in the peritoneum of a woman 6 weeks after a cesarean delivery was also described,9 this latter one misinterpreted as pseudotumoral deciduosis. Our case also occurred 2 weeks after a cesarean delivery. It is difficult however to establish a relationship between sex hormones and deciduoid occurrence.
As far as we know, only 45 cases have been reported in the literature, 22 of those described were in the pleural cavity.4 Twenty-six out of the 45 cases were female. Ages have ranged between 13 and 78 years (mean 51 years). Exposure to asbestos was documented in only 15 cases, with a mean age of onset of 59.1 years, as opposed to 47.7 years of those cases with no documented exposure to asbestos.
This variant of epithelioid mesothelioma is characterized by its unique morphology. It is composed of a proliferation of large round cells, abundant eosinophilic cytoplasm, deciduoid features and round vesicular nuclei.9 Immunohistochemical data are useful in differentiating mesotheliomas from adenocarcinomas.10 In the case that described deciduoid mesothelioma clearly expressed calretinin.
Several features described in patients with deciduoid mesothelioma are worth mentioning. The number of cases found in the pleura and in the peritoneum is balanced whereas mesothelioma in general is about 4 times more common in the pleura.11 There is a preponderance of females in deciduoid mesothelioma as opposed to the slight male predominance in mesothelioma in general. The number of young patients is significant (<40 years) in this variant of mesothelioma, such as the patient described, in contrast to the older age of patients with mesothelioma in general. Another characteristic is the lower rate of asbestos exposure in deciduoid mesothelioma. All these differences suggest that deciduoid mesothelioma may be a clinically and pathologically distinct variant.6
The best treatment option for mesothelioma is still a matter of debate and a challenge. Several studies have reported good results with EPP together with neo- or adjuvant chemotherapy.12–15 However the benefit of this multimodality therapy may have been overestimated and it should be noted that EPP is a complex procedure associated with high morbidity and mortality even during the peri-operative period, as in this case. In fact, some retrospective studies suggest that EPP offers no advantage over less radical surgical procedures such as pleurectomy and decortication16–20 that have proven effectiveness as palliative measures. Hemithoracic radiotherapy after EPP has been suggested but its role needs further evaluation. Intrapleural or local therapies, such as photodynamic therapy, hyperthermic intrapleural chemotherapy and immunotherapy, were designed to attack microscopic residual disease after surgery. However, all these modalities need further refinement. Randomized trials have shown that systemic chemotherapy is the only type of treatment to increase survival.
Our patient was started on cisplatin and pemetrexed, the standard care in the systemic therapy of mesothelioma, followed by surgical resection but died postoperatively. The cause of death was not determined.
The clinical outcome of malignant deciduoid mesothelioma is usually poor and 68% of the patients die within 1 year after starting treatment.4 However there are reported cases of patients with deciduoid mesothelioma who have survived much longer5,8 challenging the inevitability of a poor prognosis.21 More systematic studies and case reports are necessary to give a clearer picture of this variant.
In conclusion, we describe a case of deciduoid pleural mesothelioma in a 40-year-old-woman with clinical presentation 2 weeks after a cesarean delivery and with no known asbestos exposure. We want to highlight the rarity of this variant, the fact that this case is the first reported in Portugal, the role of pathology in its diagnosis, the features that distinguish deciduoid mesothelioma from mesothelioma in general and discuss the available treatment options.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Jessica Cemlyn Jones, MD, Pulmonologist, for reviewing the text.
Please cite this article as: Santos C, et al. Mesotelioma deciduóide pleural – uma entidade rara numa mulher jovem. Rev Port Pneumol. 2012. doi:10.1016/j.rppneu.2012.02.004.