Obstructive sleep apnea syndrome (OSA) is a cause of high morbidity and mortality so diagnosis and treatment is essential. Questionnaires and oximetry have been used for OSA screening.
AimTo evaluate the clinical utility of different sleep questionnaires (Stop Bang (S-B), Berlin (BQ), Epworth Sleepiness Scale (ESS)) in deciding on treatment with Continuous Positive Airway Pressure (CPAP) and to examine whether the combination of the questionnaires alone or with oximetry improves their predictive value for CPAP initiation.
MethodsPatients visiting a Sleep Clinic were prospectively studied. They completed the questionnaires. Home oximetry and in laboratory polysomnography (PSG) were performed within 3–20 days. Patients received CPAP if they were symptomatic with AHI≥5 or had AHI>15.
Results204 patients were studied (77.5%males, mean age 51.8±13.8 years, BMI 32.8±6.2kg/m2). There was a good correlation between Oxygen Desaturation Index of oximetry (ODIox) and ODI of PSG (r=0.95, p<0.0001) and between ODIox and AHI (r=0.811, p<0.0001). ODIox≥15 presented sensitivity 89.3%, specificity 83.5%, PPV 87% NPV 86.4% for CPAP initiation. ESS had the best specificity (68.6%) and PPV (68.6%) and S-B had the highest sensitivity (98%) and NPV (80%) but the lowest specificity (11%) for CPAP initiation. The combination of different questionnaires or questionnaires with oximetry did not improve their predictive value for CPAP initiation.
ConclusionsOximetry accurately predicted CPAP initiation. Questionnaires alone had limited value as screening tools for CPAP initiation; the combination of oximetry with questionnaires did not improve their predictive value.
Diagnosis and treatment of obstructive sleep apnea syndrome (OSA) is essential as it is a recognized morbidity and mortality risk factor. As the population suffering from OSA increases, there is a need for faster and less time consuming diagnosis and treatment. Oximetry has been used as a tool for diagnosing severe OSA.1,2 Several questionnaires and clinical models have been found to be accurate for the screening of high risk patients.2–6 Questionnaires improved the predictive values of portable sleep monitors (types 3 and 4) as screening tools for OSA.2,3 Additionally, limited respiratory recordings were found sufficient to titrate Continuous Positive Airway Pressure (CPAP) levels adequately in most of patients with OSA.7
The aim of this study was: (1) to examine the clinical utility of three different widely used sleep questionnaires (Epworth Sleepiness Scale (ESS), Stop Bang (S-B), Berlin Questionnaire (BQ)) as screening tools for the decision for treatment with CPAP and (2) to examine whether the combination of the questionnaires alone or with oximetry improves their predictive value for CPAP initiation in patients with suspected OSA.
Materials and methodsPatients visiting the Sleep Clinic of the Respiratory Failure Unit of G Papanikolaou Hospital (2012–2016) were prospectively studied. The inclusion criteria for the study were: patients >18 years who visited the sleep clinic for sleep problems such as snoring, witnessed apneas, daytime sleepiness. Patients were excluded if they refused to participate, were unable to use the equipment or lived >50km from the clinic, suffered from a known sleep disorder or other comorbidities (coronary heart disease, heart failure, stroke, cardiac arrhythmias, pulmonary hypertension, respiratory insufficiency, COPD, severe anemia). Informed consent was obtained from all individual participants included in the study and the protocol was approved by the Local Ethics Committee. The patients all completed three different sleep questionnaires (S-B, BQ and ESS). Body Mass Index (BMI), age, neck circumference and gender were also measured.
S-B is a quick and simple screening questionnaire for assessing the risk for OSA.8 It consists of 8 items/questions: snoring, tiredness during daytime, observed apneas, high blood pressure, BMI>35kg/m2, age (>50 years old), neck circumference (>40cm) and gender (male). Each positive answer is assigned 1 point. If the score is 0–2, there is low risk for OSA, whereas when it is >3, there is increased risk.
The BQ is a simple screening questionnaire for assessing OSA risk.9 It consists of three categories: snoring severity, exessive daytime sleepiness (EDS) and history of hypertension or obesity. If more than two categories are positive, there is high risk for OSA.
ESS is a widely used questionnaire for the assessment of EDS.10 The patients rate from 0 to 3 is the possibility of dozing off in 8 common situations. When the score is more than 10, ESS is considered abnormal (scores between 0 and 24).
Home oximetry and in laboratory polysomnography (PSG) were performed on all the participants within 3–20 days in random order. SOMNOcheck, Weinmann was used for home oximetry (only the finger pulse oximetre was used). Patients used the device at home, unsupervised, after having been trained by a sleep technician (GK). The recordings started at 12 a.m. and stopped at 6 a.m. and the data were manually scored by a technician (GK) using continuous SpO2 signal in a 2-min window display resolution. Recordings were considered adequate if the patients reported sleeping >4h; if recordings were inadequate (i.e. bad signal, recording less than 4h), a second home recording was performed. Oxygen Desaturation Index (ODI) was defined as the number of desaturations ≥4% divided by the time in bed. The cut off value of ODI≥15 was used to classify the presence of moderate to severe OSA.2
PSG recordings (Alice 5 Diagnostic Sleep System, Philips Respironics) included electroencephalography (EEG), electrooculography (EOG), electrocardiography (ECG), submental electromyography (EMG), oximetry, nasal pressure transducer and oronasal thermistor for the detection of airflow limitation, thoracic and abdominal respiratory effort bands, body position detection and microphone for the detection of snoring. PSGs were manually scored according the American Academy of Sleep Medicine (AASM) guidelines.11 Apnea Hypopnea Index (AHI) was defined as the number of apneas and hypopneas per hour of sleep. Apnea was defined as the drop of airflow ≥90% of baseline for at least 10s and hypopnea as a decrease in airflow of at least 30% for at least 10s with oxygen desaturation of ≥4% from pre-event baseline. The severity of OSA was determined by the AHI: 5–15 as mild; greater than 15–30 as moderate; greater than 30 as severe. The scoring of each patient studies (PSG, oximetry) was done by a single person who was blinded to the subject. Patients received CPAP if they had AHI>15 or AHII≥5 plus symptoms (excessive daytime sleepiness, daytime fatigue).12
Statistical analysisSPSS version 17.0 (SPSS Science, Apache Software Foundation, Chicago, IL, USA) was used. Data were presented as mean±SD unless otherwise stated. Tests were two-tailed and p<0.05 was accepted as statistically significant. Sensitivity (Se), specificity (Sp), positive predictive values (PPV), negative predictive values (NPV), positive and negative likelihood ratios (LR+/LR–) were calculated. Combined sensitivity and specificity (combination positive if all tests are positive and negative if at least one is negative) were estimated to evaluate the combination of different questionnaires with oximetry [(Se=Se1×Se2×···×Sek. Sp=1−[(1−Sp1)×(1−Sp2)×···×(1−Spk)].6 The discriminatory ability of each questionnaire or oximetry for CPAP treatment was evaluated using receiver operating characteristic (ROC) curves. The Pearson correlation coefficient was used for the assessment of possible significant correlations between different variables. Spearman's correlation coefficient was used for nonparametric variables and the chi-square for categorical variables. Separate bivariate logistic regression models were used to determine odds ratio (OR).
ResultsTwo hundred and four (204) patients were included in the study (77.5% males, mean age 51.8±13.8 years, BMI 32.8±6.2kg/m2, SaO2% awake 95.7±2). After PSG scoring, 39 (19.1%) patients did not suffer from OSA (no OSA), 29 (14.2%) suffered from mild, 54 (26.5%) from moderate and 82 (40.2%) from severe. Fourteen patients had inadequate home oximetry recordings (5 had inadequate traces for more than 50% of the recording and 9 reported sleep <4h) and repeated the home study. CPAP treatment was offered to 135 (66.2%) patients (79 with severe OSA, 51 with moderate and 6 with mild OSA). Statistically significant correlations were found between ODI of oximetry (ODIox) and ODI of PSG (r=0.95, p<0.0001), as between ODIox and AHI (r=0.811, p<0.0001).
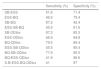
As presented in Table 1, oximetry presented high predictive values for the diagnosis of severe OSA and for AHI >15 (moderate and severe OSA) while its value was limited for mild and moderate OSA. The predictive parameters of ESS, BQ, S-B and ODIox for CPAP treatment are presented in Table 2. The ESS had the best specificity (68.6%) and PPV (68.6%), but low sensitivity (52.7%), NPV (52.7%) and OR (95% confidential interval, CI) 2.4 (1.3–4.4). S-B had the highest sensitivity (98%) and NPV (80%) but the lowest specificity (11%). BQ presented high sensitivity (89%) and NPV (71%), but lower PPV (64.5%) and specificity (35.5%). Oximetry (ODIox≥15) presented higher predictive values for CPAP treatment initiation compared with those of the questionnaires (Table 2). The combined sensitivities and specificities of the different questionnaires or of oximetry with the different questionnaires for the prediction of CPAP initiation are presented in Table 3. Neither the combination of different questionnaires nor of sleep questionnaires with oximetry improved the predictive values of oximetry alone for CPAP treatment (Tables 2 and 3).
Predictive values of ODI of oximetry ≥15 for different AHI cut-offs.
| AHI>5 plus symptoms | AHI>15 | Mild OSA | Moderate OSA | Severe OSA | |
|---|---|---|---|---|---|
| Sensitivity (%) | 69 | 82 | 3.4 | 56 | 97.5 |
| Specificity (%) | 95 | 94 | 32.5 | 43.5 | 72.6 |
| PPV (%) | 98.2 | 96.5 | 0.87 | 25.2 | 71.4 |
| NPV (%) | 42 | 72.7 | 66.2 | 74.4 | 97.7 |
| Probability (+)/(–) (%) | 56.6/43.7 | 56/44 | 28/72 | 56.3/43.7 | 56.3/43.7 |
| LR(+)/(–) | 13.4/0.32 | 14/0.2 | 0.05/2.3 | 0.99/1 | 3.56/0.03 |
| AUC (95% CI) | 0.82 (0.75–0.9) | 0.9 (0.83–0.93) | 0.18 (0.12–0.25) | 0.498 (0.4–0.59) | 0.85 (0.8–0.9) |
ODI: Oxygen Desaturation Index; AHI: Apnea Hypopnea Index; OSA: obstructive sleep apnea syndrome; PPV: positive predictive value; NPV: negative predictive value; LR: likelihood ratio; AUC: area under the ROC curve; CI: confidential interval.
Predictive parameters of ESS, Berlin, Stop-Bang and ODIox≥15 for CPAP initiation.
| ESS | Berlin | Stop-Bang | ODIox | |
|---|---|---|---|---|
| Sensitivity (%) | 52.7 | 89 | 98 | 89.3 |
| Specificity (%) | 68.6 | 35.5 | 10.7 | 83.5 |
| PPV (%) | 68.6 | 64.5 | 60.2 | 87 |
| NPV (%) | 52.7 | 71 | 80 | 86.4 |
| LR(+)/(–) | 1.7/0.7 | 1.4/0.3 | 1.1/0.2 | 5.4/0.13 |
| OR (95% CI) | 2.4 (1.3–4.4) | 4.5 (2.1–9.7) | 6 (1.2–29) | 42.2 (18.7–95) |
| AUC (95% CI) | 0.66 (0.6–0.74) | 0.6 (0.54–0.7) | 0.7 (0.6–0.78) | 0.86 (0.8–0.92) |
ESS: Epworth Sleepiness Scale; ODIox: Oxygen Desaturation Index of oximetry; CPAP: continuous positive airway pressure; PPV: positive predictive value; NPV: negative predictive value; LR: likelihood ratio; AUC: area under the ROC curve; OR: odds ratio; CI: confidential interval.
Combined sensitivities and specificities of different questionnaires or questionnaires and oximetry for CPAP initiation.
| Sensitivity (%) | Specificity (%) | |
|---|---|---|
| SB-ESS | 51.6 | 71.4 |
| ESS-BQ | 46.9 | 79.4 |
| SB-BQ | 87.2 | 42.4 |
| ESS-SB-BQ | 45.9 | 81.6 |
| SB-ODIox | 87.2 | 85.3 |
| ESS-ODIox | 46.9 | 94.8 |
| BQ-ODIox | 79.5 | 89.4 |
| ESS-SB-ODIox | 45.5 | 95.4 |
| BQ-SB-ODIox | 77.9 | 90.5 |
| BQ-ESS-ODIox | 41.9 | 96.6 |
| S-B-ESS-BQ-ODIox | 41 | 97 |
CPAP: continuous positive airway pressure; SB: Stop-Bang; ESS: Epworth Sleepiness Scale; BQ: Berlin Questionnaire; ODIox: Oxygen Desaturation Index of oximetry.
The main finding of this study was that the predictive value of questionnaires was lower than that of oximetry for CPAP treatment initiation. The combination of different questionnaires alone or with oximetry did not improve their predictive value. The predictive values of oximetry were high especially in moderate to severe disease (AHI>15). There were only 8 false positive cases, but also 28 false negative cases (17 with mild, 9 with moderate and 2 with severe OSA) either because oximetry was negative or because those patients were not eligible to initiate CPAP treatment according to our criteria. However, most of the false negative cases suffered from mild disease. Oximetry has limited predictive value for mild OSA to be treated with CPAP, only relevant in the case of symptoms or severe comorbidities. False positive/negative cases could also be explained by night to night variability.12
The predictive parameters of sleep questionnaires were worse than that of oximetry and the combination of questionnaires alone or with oximetry did not reach the predictive values of oximetry for the prediction of CPAP treatment. In the study of Nigro,13 the use of oximetry and clinical data indicated CPAP reliably in almost 90% of the population with OSA, with sensitivity ranging from 80% to 92.5% and specificity from 92% to 96% depending on the criterion used (ODI 3% or 4%). In another study,14 clinical criteria such as excessive daytime sleepiness, hypertension, arrhythmias, coronary heart disease and stroke could diagnose OSA and indicate CPAP treatment in almost 1/3 of the population who would have required CPAP after in laboratory PSG assessment and medical history. In our study, S-B had high sensitivity but very low specificity, BQ had similarly good sensitivity and better specificity than the S-B and ESS moderate specificity and rather low sensitivity. In the study of Pereira et al,15 sleep questionnaires in combination with a level III portable monitor were evaluated for identifying OSA. The objective data from the portable monitor were found to be better than questionnaires and the use of questionnaires did not increase the diagnostic utility of the portable device.15 This is similar to a previous study,6 in which we evaluated retrospectively five different questionnaires (ESS, STOP, S-B, BQ, 4 variable screening tool) for the identification of OSA patients in a sleep clinic setting. Different questionnaires were combined in order to improve their predictive value but with no significant findings.
Oximetry is as a less expensive indicator in the screening of severe OSA but not for mild disease.1,2 In one study, oximetry was found to be a useful tool especially for patients with severe disease but the predictive values of sleep questionnaires and oximetry were low in mild and moderate OSA and the combination between them did not improve their diagnostic values for OSA identification.16 However, it is important to state that the results of our study only refer to individuals without significant comorbidities, since patients with other pathologies were excluded (coronary heart disease, heart failure, stroke, cardiac arrhythmias, pulmonary hypertension, respiratory insufficiency, COPD, severe anemia) and are only significant for severe OSA.
OSA has been associated with a wide range of medical consequences including cardiovascular disease. Cardiovascular morbidity has been linked to oxygen desaturations.17,18 However, the effectiveness of CPAP in reducing the rate of cardiovascular events among patients with OSA remains controversial, even for patients with cardiovascular disease.19 Additionally OSA patients may be asymptomatic or minimally symptomatic or may have a misperception of their symptoms and this could affect the predictive value of questionnaires that are subjective. It is not clear yet, whether asymptomatic patients with mild or moderate OSA face the same cardiovascular risk as those with symptoms and whether they derive the same benefits from CPAP use.20 Additionally, symptomatic patients are the most compliant with CPAP treatment which is important for the cardiovascular benefits of the device.21
However our study has some limitations. The main limitation was that it was based on the patients visiting a sleep clinic and not on the general population. So, all the predictive values should be interpreted in this setting. Patients with severe comorbidities were excluded, as this population should not be investigated with limited recordings. However, it would be interesting to study such a population as a study by Nigro et al.22 found that a two-channel recording device (oximetry and airflow) plus clinical data (including co-morbidities as Chronic Obstructive Pulmonary Disease, Asthma, Pulmonary fibrosis, Pulmonary hypertension, Coronary arterial disease, Hypertension, Cardiac arrhythmia, Cardiac failure, Cerebrovascular disease, Diabetes mellitus, Hypothyroidism, etc.) indicated CPAP reliably in most patients with high clinical pre-test for OSA. Another important limitation of our study was that the older criteria for hypopnea scoring were used and this could have influenced our results. If the 3% desaturation threshold was employed instead of 4% and arousals were also included, the AHI would further increase and it would decrease the sensitivities of the questionnaires.23 In a previous study,13 the predictive values of the use of oximetry and clinical data for CPAP initiation were better when using the 3% desaturation threshold compared with 4%. In addition the arousal criteria were not used and so respiratory events where the drops in arterial oxygen saturation were minor but still caused arousals, were not identified as hypopneas; this would have affected the predictive values of the questionnaires.24 Additionally, all patients in our study answered the questionnaires by themselves. It would be interesting to investigate the predictive values of the questionnaires answered by their partners because it has been found that sensitivity and specificity for diagnosing OSA (AHI>15) were higher in these circumstances than when self-reported.15
In conclusion, our findings are in accordance with previous studies24 where oximetry was found reasonably accurate for predicting CPAP initiation. Questionnaires alone had limited value in predicting CPAP initiation in patients with OSA. The combination of oximetry with questionnaires did not improve their predictive value. Limited studies with oximetry could prove to be an essential tool for OSA screening, especially in high-risk populations (i.e. patients with type 2 diabetes), in which diagnosis and treatment of OSA is an important issue.25 Our results support the argument that home monitoring may replace laboratory diagnosis and treatment of OSA in situations where access to sleep studies is not available, reducing costs and facilitating rapid initiation of CPAP treatment, especially in severe disease.13,25,26 However, this may not be sufficient for all patients; PSG should be offered to symptomatic patients with negative portable monitoring, asymptomatic but highly suspected patients, to patients with co-morbidities and to those who do not improve enough with CPAP treatment. As the incidence of the OSA increases, there is an increased need for simple and economical methods13 for the diagnosis and treatment of the disease.
Author contributionAP contributed in the conception and design of the research, acquisition of data, analysis and interpretation of the data, statistical analysis, writing and drafting of manuscript, GK in sleep scoring, analysis and interpretation of the data, ED in interpretation of the data, critical revision and drafting of manuscript and PA in conception, design, critical revision and drafting of manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.