The decrease in the number of clinical trials held in Portugal shows a loss of competiveness that is disturbing. We should focus on measures that may recover the implementation of a dynamic clinical investigation field.
According to a study of the Portuguese Association of Pharmaceutical Industry (APIFARMA) published in 20131 there has been a tendency towards stagnation in the number of Clinical trials (CT) in Portugal. Nevertheless, in 2012 the market value of CT in Portugal was €36 millions. Looking at the sites where the CT were performed, between 2006 and 2011, Coimbra University Hospital Centre (CHUC) with 158 CT, North Lisbon Hospital Centre (CHLN) with 135 and São João Hospital Centre (CHSJ) with 98 were the top 3 institutions.
Compared to Cardiology with 34 CT from 2009 until 2012, Pulmonology had only 15. Compared with other countries, in 2010 Portugal had one of the lowest numbers of CT per million inhabitants, with 10 compared with Czech Republic, which achieved 47.2
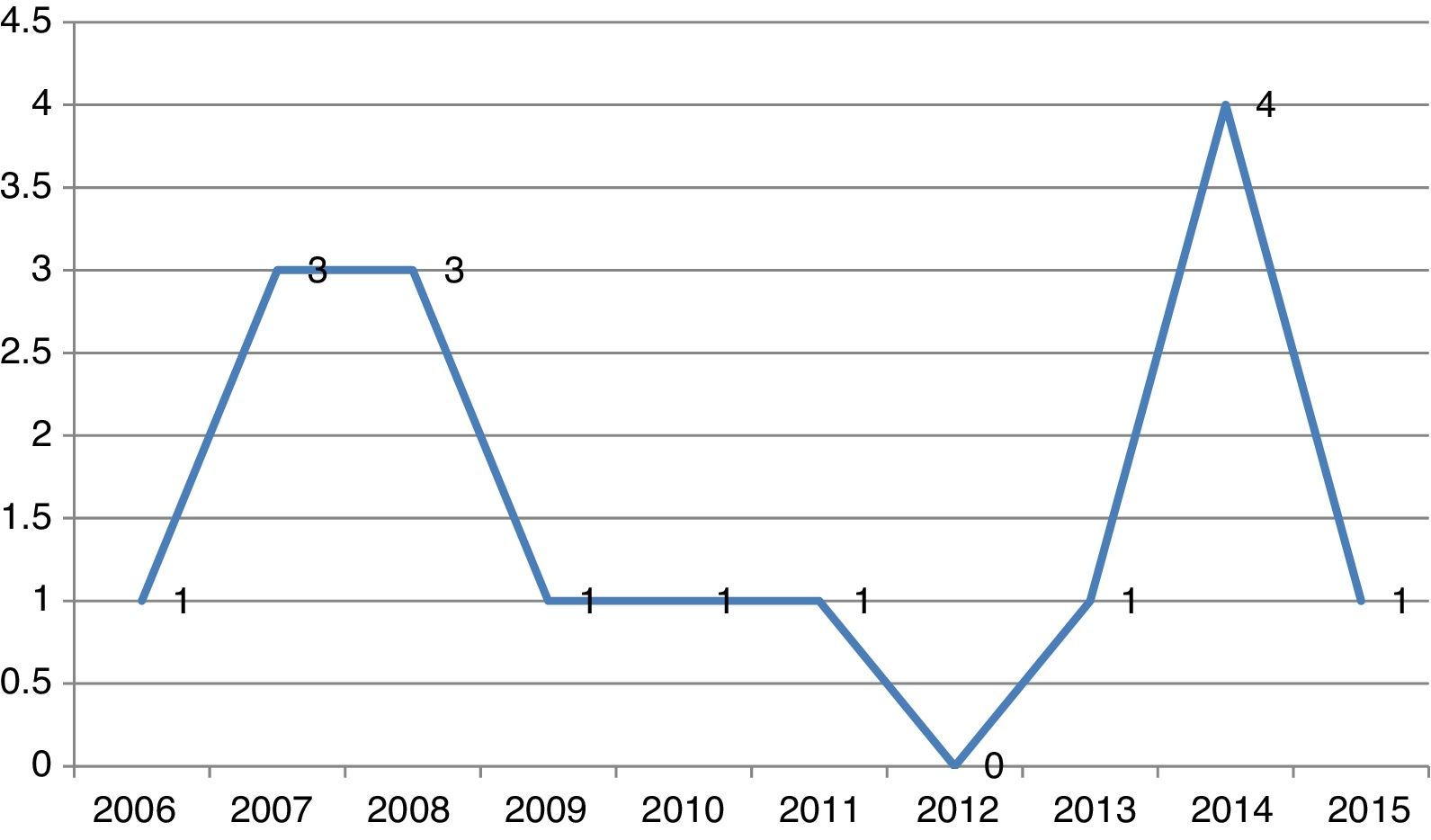
Recently the EU has developed a register for all the Clinical trials performed in Europe.3 Analysing the participation of Portuguese centres in the area of Pulmonology from 2006 to 2015, the scenario is also not very optimistic, with a total of 15 trials on COPD, 7 in Cystic Fibrosis (CF), 55 in Lung Cancer (LC) and 6 in Idiopathic Pulmonary Fibrosis (IPF). This is a long way from the involvement of Greece and the Czech Republic with, respectively, 28 and 68 CT on COPD, 4 and 13 CT on CF, 77 and 107 on LC and 6 and 12 on IPF. Moreover, analysing the participation of Portuguese centres in CT on COPD (a disease with the highest number of patients included in CT) over the last 10 years (Fig. 1), there is an irregular pattern with a minimum of 0 in 2012 and a maximum of 4 in 2014.
What can we do to improve?
By doing Clinical trials we will generate better quality data to support our decisions in healthcare.
Scientific societies should have a stronger commitment to the promotion and support of the clinical investigation in Portugal. By helping to network with European countries it will facilitate internationalization and improvement in science.
Through the creation of dedicated structures and enhancement of cooperation between Portuguese centres the results will appear in the future.
Models that include incentives for the participation of researchers and other medical professionals should be common practice. Investigator-driven CT should be also encouraged and welcomed.
If we succeed in improving these figures, the Portuguese economy will be stronger, we will increase our small footprint in the European respiratory science and our specialty will have a higher profile.
Conflicts of interestThe authors have no conflicts of interest to declare.