Pulmonary Rehabilitation Programs (PRP) have been shown to improve exercise capacity and health status and to reduce dyspnea and use of healthcare resources, in patients with chronic lung disease. These benefits usually wane after the programs.
AimEvaluate functional capacity and health status 2 years after the end of a PRP.
MethodsRetrospective study of patients who took part in PRP. After PRP, patients who reported a physically active lifestyle were included in the active group (AG). The other patients were considered as the control group (CG). Functional capacity was evaluated with six-minute walk distance (6MWD) and health status with St. George's Respiratory Questionnaire (SGRQ).
ResultsThirty-two patients were included, 24 in the AG and 8 in the CG. Immediately after PRP, there was a significant improvement in the 6MWD and SGRQ global score, for both groups. After completing PRP, in the AG, there was a decline in the mean 6MWD when evaluated at 6 months, 1 and 2 years and also in health status. However, after 2 years, the AG continued to show an average improvement of 32m (p=0.03) in the 6MWD and at least 4 points in SGRQ compared to pre-PRP, while in the CG, there was a clinically significant decline in 6MWD (−34m) and SGRQ score (13 points worse).
ConclusionDespite the progressive decline of benefits gained after completing PRP, in the AG these are still significantly positive after 2 years. An active lifestyle seems to help maintain the benefits of the Rehabilitation Program.
Os programas de reabilitação respiratória (PRR) têm demonstrado em doentes com patologia pulmonar crónica, melhoria da capacidade de exercício e do estado de saúde e diminuição da dispneia e da utilização de recursos de saúde. Habitualmente, estes benefícios diminuem após conclusão dos programas.
ObjetivoAvaliar a capacidade funcional e o estado de saúde 2 anos após o término de um PRR.
MétodosEstudo retrospetivo de doentes que completaram um PRR. Após o PRR, os doentes que referiam ter adotado um estilo de vida fisicamente ativo foram incluídos no grupo ativo (GA). Os restantes doentes foram considerados como grupo controlo (GC). A capacidade funcional foi avaliada com a prova de marcha dos 6 minutos (PM6m) e o estado de saúde com o questionário de St. George na doença respiratória (SGRQ).
ResultadosForam incluídos 32 doentes, 24 no GA e 8 no GC. Imediatamente após o PRR observou-se, em ambos os grupos, uma melhoria significativa na PM6m e na pontuação total do SGRQ. Após o término do PRR, no GA, observou-se um declínio na distância média percorrida na PM6m aos 6 meses, 1 ano e 2 anos, bem como no estado de saúde.
Contudo, 2 anos após o PRR e comparando com os valores avaliados antes do início do PRR, o GA continuava a apresentar uma melhoria na distância percorrida na PM6m, em média de 32m (p=0,03) e de pelo menos 4 pontos no SGRQ. No GC observou-se um declínio clinicamente significativo na PM6m (-34m) e no SGRQ (agravamento de 13 pontos).
ConclusãoEmbora se verifique uma perda progressiva dos benefícios do PRR após a sua cessação, estes ainda são significativamente positivos até 2 anos após o treino no GA. Um estilo de vida fisicamente ativo parece contribuir para manter os benefícios do Programa de Reabilitação.
There is a significant body of evidence to support the efficacy of Pulmonary Rehabilitation Programs (PRP) in patients with chronic respiratory disease, mostly COPD.1–5
PRP with exercise training over at least 8 weeks have been shown to improve exercise performance, health status, dyspnea and to reduce use of health care resources.6,7 These benefits tend to decline after the intensive phase of the program.7
The fact that the benefits revert after training ceases3,8 justifies the implementation of procedures to maintain the PRP benefits, especially through improving long-term self-management and adherence to exercise regimen in the home.9
Several strategies have been developed to maintain the PRP benefits, such as telephone calls, support groups, regular visits to the rehabilitation center, regular home visits and activity monitors.7,9,10 However, the results of these interventions vary among the studies.
The purpose of this study was to evaluate functional capacity and health status at 6 months, 1 and 2 years after conclusion of a PRP in patients regularly followed in respiratory failure day-hospital and to evaluate a potential relationship between the maintenance of physical activity and the follow-up outcomes.
MethodsSubjectsPatients admitted to a Pulmonary Rehabilitation Program at respiratory failure day-hospital of Pulido Valente Hospital from September 2005 to February 2010 were included. Eligibility criteria included all patients who completed the PRP during this period and who agreed to participate in the follow-up program.
Study designThe Pulmonary Rehabilitation Program lasted at least 12 weeks and was composed of exercise training (treadmill or cycling exercises 3 days per week) with target intensity of 80% of the peak work rate reached during a previous maximal incremental exercise test on the same ergometer. The length of the program was what was needed to reach the target intensity of 80% of peak work rate. Some patients had a slower progression, due to symptoms and some of them had to interrupt the program due to exacerbations, but resumed it as soon as possible.
The training modality was according to patient preference and to the probability that patient would maintain this exercise after ending PRP.
All the patients also received educational sessions and promotion of self management skills, psychosocial and nutritional support, if needed, and other supervised exercises (e.g. breathing exercises and upper extremity training). Along with the program, the patients were encouraged to adopt an active lifestyle. Six months after the PRP, patients were questioned about their usual activity level. If they reported regular exercise, as prescribed (e.g. at least 30minutes walks or riding a stationary bicycle 3 times a week) they were included in active group (AG), if instead they became sedentary again, they were considered as control group (CG). Both patient groups were encouraged to continue exercise in each assessment, every 6 months.
The medical records of patients who fulfilled the inclusion criteria were reviewed and were analyzed according to demographic data, smoking history, pulmonary disease, comorbidities (Charlson index11–13), anxiety and depression levels (evaluated by the Hospital Anxiety and Depression Scale14), Medical Research Council (MRC) score of dyspnea,15 lung function tests, PRP duration, target intensity achieved during PRP, six-minute walk test (6MWT)16 and St. George's Respiratory Questionnaire Disease (SGRQ).17 All the patient data were encoded in order to ensure confidentiality.
The 6MWT was performed according to the American Thoracic Society Guidelines.16 To overcome the learning effect, 6MWT was initially performed three times, and the third test was considered the baseline 6MWT. Our protocol included 6MWT at baseline (before the PRP), immediately after PRP, at 6 months, at 1 year and at 2 years after PRP. We considered 25 meter as the minimal clinically important difference for 6MWD, as recently evidenced by Holland and colleagues.18
SGRQ is a standardized self-completion questionnaire to evaluate impaired health status in patients with respiratory disease, the total score zero indicates the best result and 100 the worst.17,19 SGRQ was performed before PRP, immediately following PRP, at 1 and 2 years after PRP. We considered 4 points as minimal clinically important difference as stated by Paul Jones.19
Statistical analysisData are presented as mean±standard deviation for continuous variables and frequencies and percentages for categorical variables.
All variables were tested for normal distribution by frequency histogram and Kolmogorov–Smirnov test or Shapiro–Wilk test. The difference between two means was determined using Student's t-test or Mann–Whitney test when appropriate.
Proportions and categorical variables were analyzed with Chi-square test or Fisher's exact test when appropriate. A p-value<0.05 was considered statistically significant.
All statistical analyses were performed using PASW software (version 18; SPSS Inc., Chicago, IL, USA).
ResultsA total of 32 patients were enrolled in the study, 84.4% males, with a mean age of 66.8±9.4 years. Most patients were ex-smokers (90.6%) and had a diagnosis of COPD (81.2%). The majority of the COPD patients were in GOLD stage IV (80.8%). The mean duration of the PRP was 26 weeks and all the patients achieved the target intensity (80% of peak work rate previously evaluated). The majority of patients trained on treadmill (65.6%) and 34.4% in cycle ergometer.
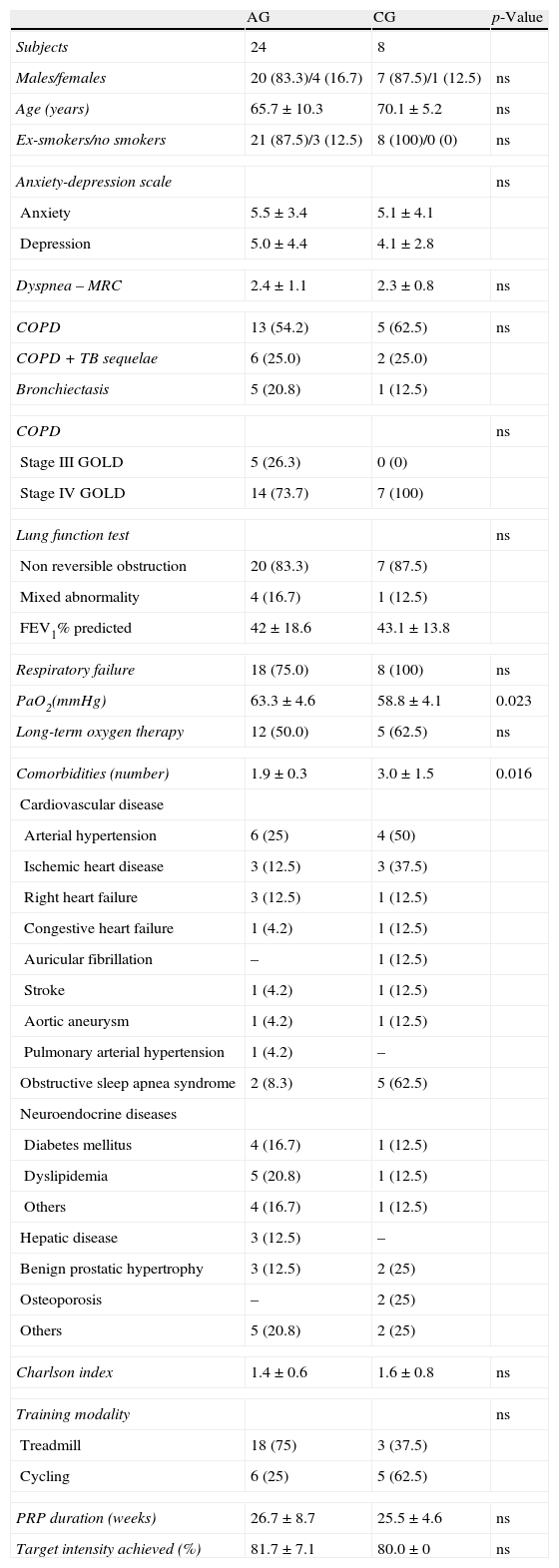
24 patients were included in the active group (AG) and 8 patients in the control group (CG). Patient baseline characteristics were similar in both groups (Table 1), except that CG were more hypoxemic (58.8mmHg versus 63.3mmHg, p=0.023) and had more comorbidities than AG. In the AG the mean number of comorbidities was 1.9 and the most common were cardiovascular diseases (41.7%), followed by dyslipidemia (20.8%) and diabetes (16.7%). The CG patients had a mean of 3 comorbidities; the most frequent were cardiovascular diseases (75%) and sleep apnea-hypopnea syndrome (62.5%) (Table 1).
Clinical and demographic characteristics of the two groups.
| AG | CG | p-Value | |
| Subjects | 24 | 8 | |
| Males/females | 20 (83.3)/4 (16.7) | 7 (87.5)/1 (12.5) | ns |
| Age (years) | 65.7±10.3 | 70.1±5.2 | ns |
| Ex-smokers/no smokers | 21 (87.5)/3 (12.5) | 8 (100)/0 (0) | ns |
| Anxiety-depression scale | ns | ||
| Anxiety | 5.5±3.4 | 5.1±4.1 | |
| Depression | 5.0±4.4 | 4.1±2.8 | |
| Dyspnea – MRC | 2.4±1.1 | 2.3±0.8 | ns |
| COPD | 13 (54.2) | 5 (62.5) | ns |
| COPD+TB sequelae | 6 (25.0) | 2 (25.0) | |
| Bronchiectasis | 5 (20.8) | 1 (12.5) | |
| COPD | ns | ||
| Stage III GOLD | 5 (26.3) | 0 (0) | |
| Stage IV GOLD | 14 (73.7) | 7 (100) | |
| Lung function test | ns | ||
| Non reversible obstruction | 20 (83.3) | 7 (87.5) | |
| Mixed abnormality | 4 (16.7) | 1 (12.5) | |
| FEV1% predicted | 42±18.6 | 43.1±13.8 | |
| Respiratory failure | 18 (75.0) | 8 (100) | ns |
| PaO2(mmHg) | 63.3±4.6 | 58.8±4.1 | 0.023 |
| Long-term oxygen therapy | 12 (50.0) | 5 (62.5) | ns |
| Comorbidities (number) | 1.9±0.3 | 3.0±1.5 | 0.016 |
| Cardiovascular disease | |||
| Arterial hypertension | 6 (25) | 4 (50) | |
| Ischemic heart disease | 3 (12.5) | 3 (37.5) | |
| Right heart failure | 3 (12.5) | 1 (12.5) | |
| Congestive heart failure | 1 (4.2) | 1 (12.5) | |
| Auricular fibrillation | – | 1 (12.5) | |
| Stroke | 1 (4.2) | 1 (12.5) | |
| Aortic aneurysm | 1 (4.2) | 1 (12.5) | |
| Pulmonary arterial hypertension | 1 (4.2) | – | |
| Obstructive sleep apnea syndrome | 2 (8.3) | 5 (62.5) | |
| Neuroendocrine diseases | |||
| Diabetes mellitus | 4 (16.7) | 1 (12.5) | |
| Dyslipidemia | 5 (20.8) | 1 (12.5) | |
| Others | 4 (16.7) | 1 (12.5) | |
| Hepatic disease | 3 (12.5) | – | |
| Benign prostatic hypertrophy | 3 (12.5) | 2 (25) | |
| Osteoporosis | – | 2 (25) | |
| Others | 5 (20.8) | 2 (25) | |
| Charlson index | 1.4±0.6 | 1.6±0.8 | ns |
| Training modality | ns | ||
| Treadmill | 18 (75) | 3 (37.5) | |
| Cycling | 6 (25) | 5 (62.5) | |
| PRP duration (weeks) | 26.7±8.7 | 25.5±4.6 | ns |
| Target intensity achieved (%) | 81.7±7.1 | 80.0±0 | ns |
Data were presented in number (%) and mean±SD.
AG: active group; CG: control group; ns:not statistically significant; MRC: Medical Research Council; COPD: chronic obstructive pulmonary disease; TB: tuberculosis; GOLD: global initiative for chronic obstructive lung disease; FEV1: forced expiratory volume in 1s; PRP: Pulmonary Rehabilitation Program.
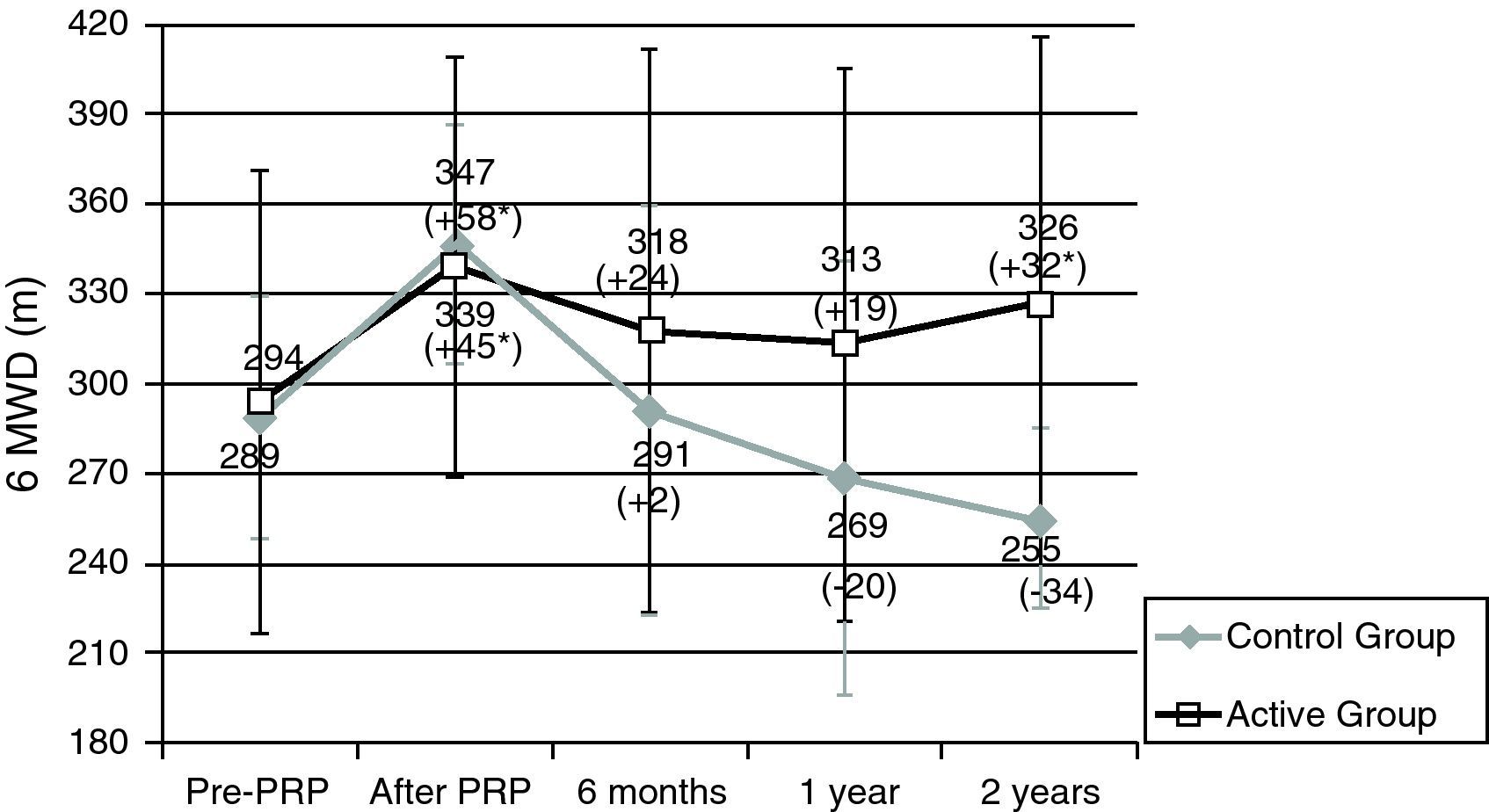
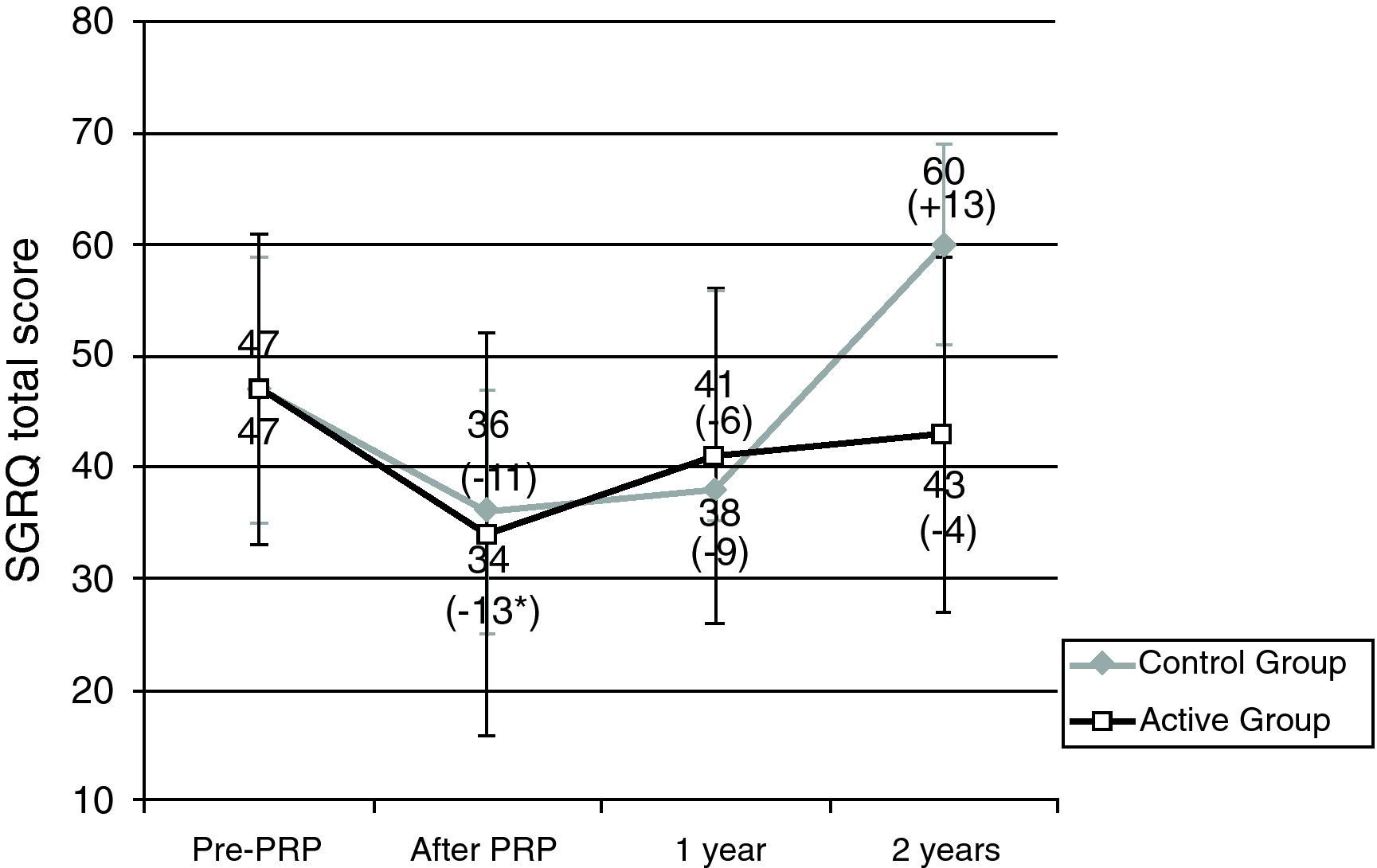
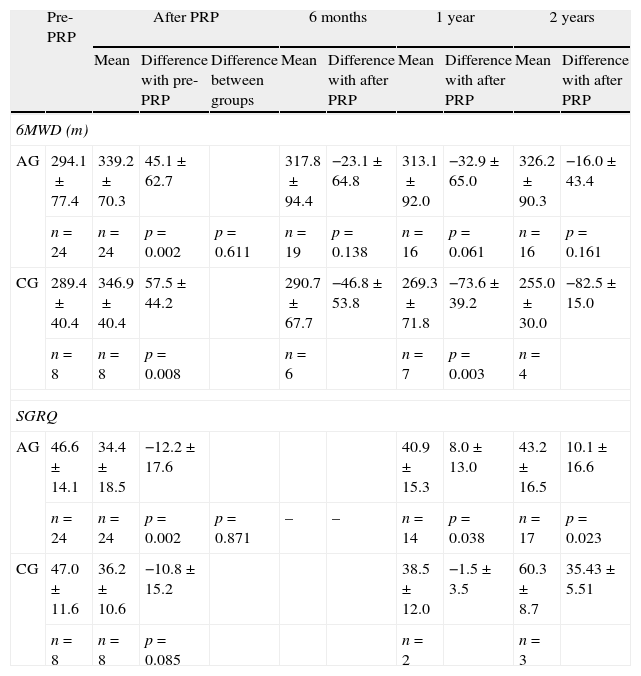
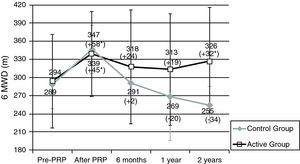
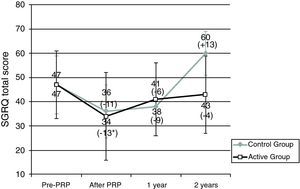
Patients in the AG and in the CG had similar results in 6MWT and in SGRQ before PRP (Table 2, Figs. 1 and 2). All subjects showed a significant improvement in the mean six-minute walk distance (6MWD) (Fig. 1) and total SGRQ score (Fig. 2) immediately after PRP. AG had an improvement of 45.1m in 6MWD (p=0.002) and 12.2 points in total SGRQ score (p=0.002) (Table 2, Figs. 1 and 2). Patients in CG had an improvement of 57.5m in the 6MWD (p=0.008) and 10.8 points less in total SGRQ score (p=0.085) (Table 2, Figs. 1 and 2). There were no significant differences between the two groups.
Exercise capacity and health status.
| Pre-PRP | After PRP | 6 months | 1 year | 2 years | ||||||
| Mean | Difference with pre-PRP | Difference between groups | Mean | Difference with after PRP | Mean | Difference with after PRP | Mean | Difference with after PRP | ||
| 6MWD (m) | ||||||||||
| AG | 294.1±77.4 | 339.2±70.3 | 45.1±62.7 | 317.8±94.4 | −23.1±64.8 | 313.1±92.0 | −32.9±65.0 | 326.2±90.3 | −16.0±43.4 | |
| n=24 | n=24 | p=0.002 | p=0.611 | n=19 | p=0.138 | n=16 | p=0.061 | n=16 | p=0.161 | |
| CG | 289.4±40.4 | 346.9±40.4 | 57.5±44.2 | 290.7±67.7 | −46.8±53.8 | 269.3±71.8 | −73.6±39.2 | 255.0±30.0 | −82.5±15.0 | |
| n=8 | n=8 | p=0.008 | n=6 | n=7 | p=0.003 | n=4 | ||||
| SGRQ | ||||||||||
| AG | 46.6±14.1 | 34.4±18.5 | −12.2±17.6 | 40.9±15.3 | 8.0±13.0 | 43.2±16.5 | 10.1±16.6 | |||
| n=24 | n=24 | p=0.002 | p=0.871 | – | – | n=14 | p=0.038 | n=17 | p=0.023 | |
| CG | 47.0±11.6 | 36.2±10.6 | −10.8±15.2 | 38.5±12.0 | −1.5±3.5 | 60.3±8.7 | 35.43±5.51 | |||
| n=8 | n=8 | p=0.085 | n=2 | n=3 | ||||||
Data were presented in mean±SD.
AG: active group; CG: control group; PRP: Pulmonary Rehabilitation Program; 6MWD: six-minute walk distance; SGRQ: St. George's Respiratory Questionnaire.
Results for six-minute walk distance (6MWD). Data are represented as mean value±SD.
PRP: Pulmonary Rehabilitation Program; Pre: before pulmonary rehabilitation. *Significant improvement after rehabilitation (p<0.05). Difference (in m) After – Pre-PRP appears in brackets.
Sample sizes: 24, 24, 19, 16, 16 for pre-PRP, after PRP, 6 months, 1 year and 2 years, respectively, for the active group; 8, 8, 6, 7, 4 for pre-PRP, after PRP, 6 months, 1 year and 2 years, respectively, for the control group.
Results for St. George's Respiratory Questionnaire (SGRQ). Data are represented as mean value±SD.
PRP: Pulmonary Rehabilitation Program; Pre: before pulmonary rehabilitation. *Significant improvement after rehabilitation (p<0.05). Difference (in m) After – pre-PRP appears in brackets.
Sample sizes: 24, 24, 14, 17 for pre-PRP, after PRP, 1 year and 2 years, respectively, for the active group; 8, 8, 2, 3 for pre-PRP, after PRP, 1 year and 2 years, respectively, for the control group.
After PRP, in AG there was a decline in mean 6MWD at 6 months (−23.1m), 1 year (−32.9m) and 2 years (−16.0m) (Table 2). However these patients always showed a better result in mean 6MWD than before PRP (Fig. 1) and 2 years after PRP, this improvement was still clinically (+32m) and statistically significant (p=0.034). Two years after PRP, 69% of the patients had a better or an equal result in the 6MWT than before PRP.
In the CG after completing PRP, there was a tendency for a sharper decline in mean 6MWD at 6 months (−46.8m), 1 year (−73.6m) and 2 years (−82.5m) (Table 2). After 2 years 6MWD was in average, 34m less than before the PRP (Fig. 1).
At the end of PRP there was an improvement in health status in both groups. At 1 year and 2 years after PRP, in AG mean SGRQ was 40.9 points and 43.2 points, respectively, higher than values obtained immediately after PRP; however health status continued to be better than values obtained before PRP (Table 2 and Fig. 2). Two years after PRP, 65% of patients had a better result in SGRQ than before PRP. On the contrary, in CG there were changes in total SGRQ score, with a tendency to get worst especially 2 years after PRP, where the mean SGRQ score was 60.3 points (13.3 points higher than before PRP) (Table 2 and Fig. 2).
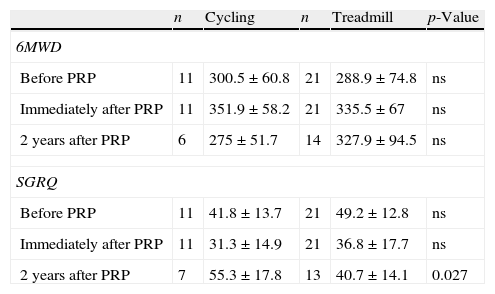
Looking at training modalities, the results in the 6MWD and SGRQ at short-term were similar for cycling and treadmill. However, 2 years after PRP, patients that trained in cycle ergometer had a worst result in SGRQ (55.3 points) than patients that trained on treadmill (40.7 points) (p=0.027) (Appendix, Table A1). Compared to the AG (25%), there were a higher number of patients training in cycle ergometer (62.5%) in CG, however this was not statistically significant.
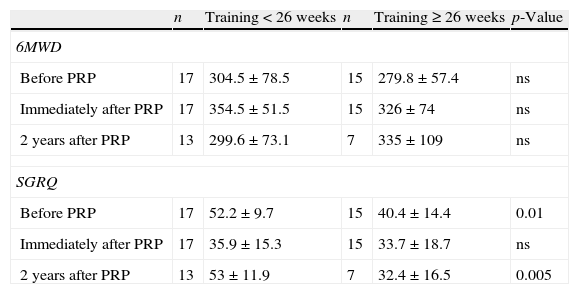
Considering 26 weeks as a cut-off for duration of PRP, there was no difference in 6MWD and SGRQ at short-term. However 2 years after PRP, patients that had a training lasting less than 26 weeks had a worst result in SGRQ, than patients with a lengthier training (p=0.005) (Appendix, Table A2).
There were no significant differences in mean 6MWT and SGRQ related to gender and pulmonary pathology, as for the presence of TB sequelae or bronchiectasis. Also, there were no correlation between age or Charlson index and results obtained at the 6MWT and SGRQ (data not shown).
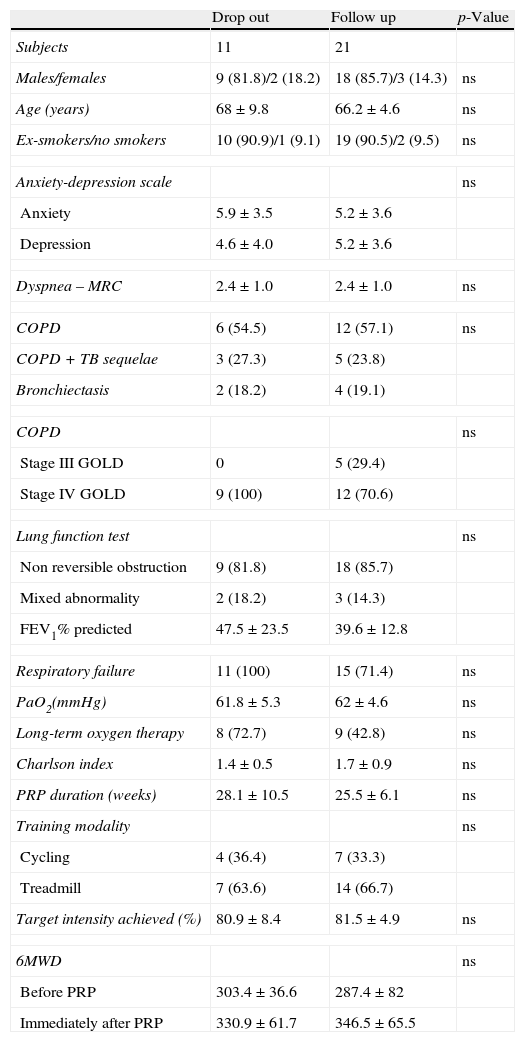
Throughout the study, there were 7 follow up appointments missing in the AG (26%) and 4 in the CG (50%) (Figs. 1 and 2). Baseline clinical and demographic characteristics were similar in patients that completed follow up and patients that dropped out (Appendix, Table A3).
DiscussionThe benefits of the PRP in the management of patients with chronic respiratory disease already known and well-established,1–5 were also observed in this study. In both groups, after PRP, patients showed a clinical and statistically significant improvement in health status and functional capacity.
In our study all the patients achieved a target intensity of 80% of peak work rate, which is different from what Maltais et al.20 reported, where only 11.9% of moderate and severe COPD patients reached this target intensity after 12 weeks of training. All our patients achieved the intensity of 80%, because the PRP length was adjusted to reach this target. High intensity training is related with more pronounced physiological adaptation (less ventilation requirements, reduction in lactate acidosis and greater improvements in exercise endurance) as evidenced by Casaburi et al.21
To the best of our knowledge this is the first study where most patients with severe and very severe COPD maintain improvement in 6MWD and health status 2 years after PRP, when compared to pre-PRP values.
In our study, program duration and location might have influenced the good outcomes evidenced after Pulmonary Rehabilitation Program and subsequent compliance of AG. The length of our PRP is longer than usual (around 26 weeks), due to patient's severity and advanced age. Other studies evidenced that longer programs seem to enhance long-term effects.2,22 We believe that longer PRP with a close contact between patients and their carers and the multidisciplinary team in an outpatient day-hospital leads to rehabilitation outcomes.
Heppner et al.23 also found that regular exercise after completing pulmonary rehabilitation is associated with slower declines in overall health status and walking self-efficacy as well as reducing progression of dyspnea during activities of daily living. However their COPD patients were less severe (moderate to severe).
In a previous study of Spencer et al. showed that a supervised weekly outpatient-based exercise program was able to maintain patients exercise capacity and health status for 12 months.7 In their study, the control group, who performed unsupervised home exercise, also maintained those benefits. Their control group was similar to our active group and the long-term results were similar, however our patients were more severe (Spencer et al. studied moderate severity COPD patients)7 and maintained these benefits for 2 years.
In another study, Brooks et al.9 compared the effects of two post rehabilitation programs on functional capacity and health status in severe COPD patients. In one group, patients attended monthly group sessions and every 2 weeks received a phone call from a physical therapist; in the other group, patients visited the physical therapist every 3 months. As in our study, a significant improvement was observed in the 6MWT and in the SGRQ after the rehabilitation program. However, in this study, there was a clear deterioration in functional capacity and health status 12 months after completion of pulmonary rehabilitation, in both groups.
On the other hand, poor adherence has a detrimental effect on morbidity, mortality and health care resources.9 We do not have a clear explanation for the poor adherence of control group to an active lifestyle, as both groups were similar in terms of baseline clinical and demographic characteristics and similar magnitude of PRP benefits in both groups (Table 1). Translation of the acquired skills to patients lifestyle may be prevented by different barriers:lack of self confident, cognitive impairment and behavioral issues, lack of motivation and the presence of physical barriers at home or in the community.24,25
The CG poor adherence to follow-up testing could be explained by the fact they were also less compliant to exercise.
A possible explanation for the worst long-term results with patients that did cycling during PRP, could be the difficulty of maintaining this exercise regularly at home and consequently they became less active.
Although mean PaO2 was lower in CG (p=0.023) which might represent a more disabled condition, there was no significant difference in the number of patients on long-term oxygen therapy (Table 1). The CG had a higher number of comorbidities (3.0 versus 1.9, p=0.016), however Charlson index was similar in both groups.
Age, gender, and the presence of tuberculous sequelae or bronchiectasis did not seem to affect the results either.
We believe that poor adherence to exercise might have caused deterioration of CG health condition with subsequent reduction in functional capacity and health status.
Limitations of our study include the small size of our sample with further reduction due to dropouts and being a retrospective study.
Although self-report of physical activity could be a possible bias, as we could not confirm the patients level of activity, the results show concordance of better outcomes in the group that reports maintaining regular exercise.
The outcomes of this study point to the need of further research in this area, in order to identify prognostic features associated with long-term success or failure in pulmonary rehabilitation and the development of strategies to maintain the benefits of rehabilitation.
ConclusionA Pulmonary Rehabilitation Program of about 26 weeks in a respiratory failure day-hospital in severe and very severe chronic lung disease patients provided significant benefits on functional capacity and health status. Those outcomes decreased after completion of the program, but remained significantly positive 2 years later, in patients that were motivated and compliant to exercise.
Regular exercise after rehabilitation may be protective against progressive loss of functional capacity and health status in severe and very severe chronic lung disease patients.
Although the sample is small, this study evidenced the need to implement strategies that ensure maintenance of Pulmonary Rehabilitation benefits.
Conflicts of interestThe authors have no conflicts of interest to declare.
To Dr. Manuela Zamith and Dr. Paulo Nicola for the comments derived from the review of this work.
Training modality and results in 6MWD and SGRQ.
| n | Cycling | n | Treadmill | p-Value | |
| 6MWD | |||||
| Before PRP | 11 | 300.5±60.8 | 21 | 288.9±74.8 | ns |
| Immediately after PRP | 11 | 351.9±58.2 | 21 | 335.5±67 | ns |
| 2 years after PRP | 6 | 275±51.7 | 14 | 327.9±94.5 | ns |
| SGRQ | |||||
| Before PRP | 11 | 41.8±13.7 | 21 | 49.2±12.8 | ns |
| Immediately after PRP | 11 | 31.3±14.9 | 21 | 36.8±17.7 | ns |
| 2 years after PRP | 7 | 55.3±17.8 | 13 | 40.7±14.1 | 0.027 |
SGRQ: St. George's Respiratory Questionnaire; 6MWD: six-minute walk distance; PRP: Pulmonary Rehabilitation Program; ns:not statistically significant.
Training duration and results in 6MWD and SGRQ.
| n | Training<26 weeks | n | Training≥26 weeks | p-Value | |
| 6MWD | |||||
| Before PRP | 17 | 304.5±78.5 | 15 | 279.8±57.4 | ns |
| Immediately after PRP | 17 | 354.5±51.5 | 15 | 326±74 | ns |
| 2 years after PRP | 13 | 299.6±73.1 | 7 | 335±109 | ns |
| SGRQ | |||||
| Before PRP | 17 | 52.2±9.7 | 15 | 40.4±14.4 | 0.01 |
| Immediately after PRP | 17 | 35.9±15.3 | 15 | 33.7±18.7 | ns |
| 2 years after PRP | 13 | 53±11.9 | 7 | 32.4±16.5 | 0.005 |
SGRQ: St. George's Respiratory Questionnaire; 6MWD: six-minute walk distance; PRP: Pulmonary Rehabilitation Program; ns:not statistically significant.
Baseline characteristics of patients that completed follow-up and patients that dropped-out.
| Drop out | Follow up | p-Value | |
| Subjects | 11 | 21 | |
| Males/females | 9 (81.8)/2 (18.2) | 18 (85.7)/3 (14.3) | ns |
| Age (years) | 68±9.8 | 66.2±4.6 | ns |
| Ex-smokers/no smokers | 10 (90.9)/1 (9.1) | 19 (90.5)/2 (9.5) | ns |
| Anxiety-depression scale | ns | ||
| Anxiety | 5.9±3.5 | 5.2±3.6 | |
| Depression | 4.6±4.0 | 5.2±3.6 | |
| Dyspnea – MRC | 2.4±1.0 | 2.4±1.0 | ns |
| COPD | 6 (54.5) | 12 (57.1) | ns |
| COPD+TB sequelae | 3 (27.3) | 5 (23.8) | |
| Bronchiectasis | 2 (18.2) | 4 (19.1) | |
| COPD | ns | ||
| Stage III GOLD | 0 | 5 (29.4) | |
| Stage IV GOLD | 9 (100) | 12 (70.6) | |
| Lung function test | ns | ||
| Non reversible obstruction | 9 (81.8) | 18 (85.7) | |
| Mixed abnormality | 2 (18.2) | 3 (14.3) | |
| FEV1% predicted | 47.5±23.5 | 39.6±12.8 | |
| Respiratory failure | 11 (100) | 15 (71.4) | ns |
| PaO2(mmHg) | 61.8±5.3 | 62±4.6 | ns |
| Long-term oxygen therapy | 8 (72.7) | 9 (42.8) | ns |
| Charlson index | 1.4±0.5 | 1.7±0.9 | ns |
| PRP duration (weeks) | 28.1±10.5 | 25.5±6.1 | ns |
| Training modality | ns | ||
| Cycling | 4 (36.4) | 7 (33.3) | |
| Treadmill | 7 (63.6) | 14 (66.7) | |
| Target intensity achieved (%) | 80.9±8.4 | 81.5±4.9 | ns |
| 6MWD | ns | ||
| Before PRP | 303.4±36.6 | 287.4±82 | |
| Immediately after PRP | 330.9±61.7 | 346.5±65.5 | |
Data were presented in number (%) and mean±SD.
NS: not statistically significant; MRC: Medical Research Council; COPD: chronic obstructive pulmonary disease; TB: tuberculosis; GOLD: global initiative for chronic obstructive lung disease; FEV1: forced expiratory volume in 1s; PRP: Pulmonary Rehabilitation Program; 6MWD: six-minute walk distance.
Please cite this article as: Areias V, et al. Evolução da capacidade funcional e estado de saúde 2 anos após um programa de reabilitação respiratória. Rev Port Pneumol. 2012. http://dx.doi.org/10.1016/j.rppneu.2012.02.010.