There are data about the immunomodulatory properties of some macrolides in cryptogenic organizing pneumonia (COP) as an alternative to corticosteroids in mild disease or as adjuvant to standard therapy.
A sixty-year-old female, with a controlled intrinsic asthma, presented with COP and recurrent respiratory exacerbations despite corticosteroid and immunossupressant therapy. Azithromycin (500mg, on alternate days) as an adjuvant to steroids was then started, with clinical and functional improvement and regression of lung infiltrates. Withdrawal of steroids was possible in one year, without evidence of relapse in the next six months. Azithromycin was maintained (three times per week) with no documentation of adverse side effects.
This clinical case reinforces the potential role of macrolides anti-inflammatory properties in COP as corticosteroids adjuvant therapy.
Existem dados na literatura sobre o uso das propriedades imunomoduladoras de alguns macrólidos no tratamento da pneumonia organizativa criptogénica (COP) como alternativa aos corticoesteróides na doença ligeira ou como adjuvantes da terapêutica padrão.
Os autores descrevem o caso de uma mulher de 60 anos de idade, com asma intrínseca controlada, que apresentou uma COP e exacerbações respiratórias de repetição, apesar da corticoterapia e terapêutica imunossupressora instituídas. Após início de azitromicina (500mg, dias alternados), como adjuvante da corticoterapia, verificou-se melhoria clínica e funcional e regressão dos infiltrados pulmonares. A suspensão dos corticoesteróides foi possível no período de um ano, sem evidência de recidiva nos seis meses seguintes. A azitromicina foi mantida (3 vezes/semana) sem documentação de efeitos laterais adversos.
Este caso clínico reforça o potencial papel das propriedades anti-inflamatórias dos macrólidos na COP, como terapêutica adjuvante dos corticoesteróides.
Cryptogenic organising pneumonia (COP) is an inflammatory disease that mainly affects the alveolar airspaces, ducts and small airways, although the interstitium can also be involved.1 The histological pattern is that of organizing pneumonia (OP), which can be seen in a wide variety of settings. The term COP is used when the disease is idiopathic.1Corticosteroids are the first line therapy in the majority of patients, these are usually effective and lead to a good prognosis.2,3 There are, however, some reports of patients responding to the immunomodulatory properties of chronic low dose therapy with macrolides, providing an alternative in patients with mild disease or for those who cannot tolerate steroids or as adjuvant to standard treatment.4–9
The authors report a case of a sixty-year-old woman, with controlled intrinsic bronchial asthma, who presented COP and several respiratory exacerbations despite corticosteroid and immunossupressant therapy, being successfully treated with azithromycin as an adjuvant to steroids. A brief review on the literature data about the anti-inflammatory effects of macrolides in chronic airway diseases is included.
Case reportA sixty-year-old female with a controlled intrinsic bronchial asthma (intermittent), diagnosed in childhood, presented with recurrent pneumonias which had led to multiple hospital admissions. She had never smoked and worked as financial administrator.
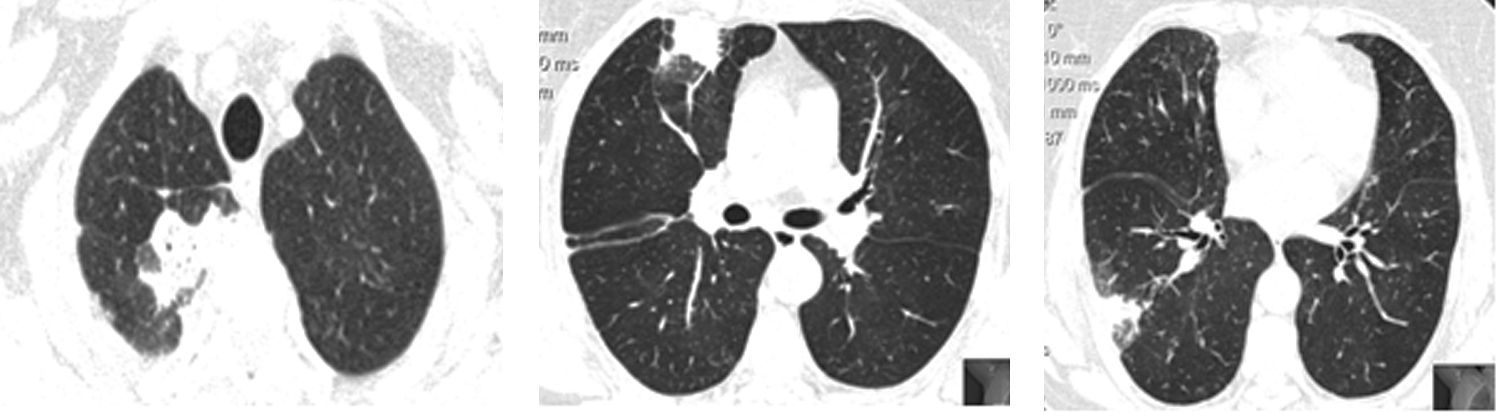
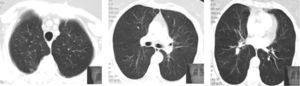
In the previous five years, as well as chronic treatment with a long acting bronchodilator and corticosteroid (salmeterol 50/fluticasona 250μg), she had had multiple courses of systemic corticosteroids and antibiotics due to respiratory exacerbations consisting of fever, wheeze, dyspnea and sometimes pleuritic chest pain. During these episodes, chest high resolution computed tomography (HRCT) showed peripheral and migratory multifocal consolidations with air bronchogram and a ground glass pattern, sometimes without being completely resolved between crises (Fig. 1). Bronchoalveolar lavage (BAL) performed during one hospital admission, revealed neutrophilia (12.8%) and a slight eosinophilia (2.2%); there was no lymphocytosis (13.6%). No microbiological agents or evidence of malignancy were found. Autoimmunity and serologic blood studies did not point to any specific etiology. Transthoracic core biopsy was inconclusive.
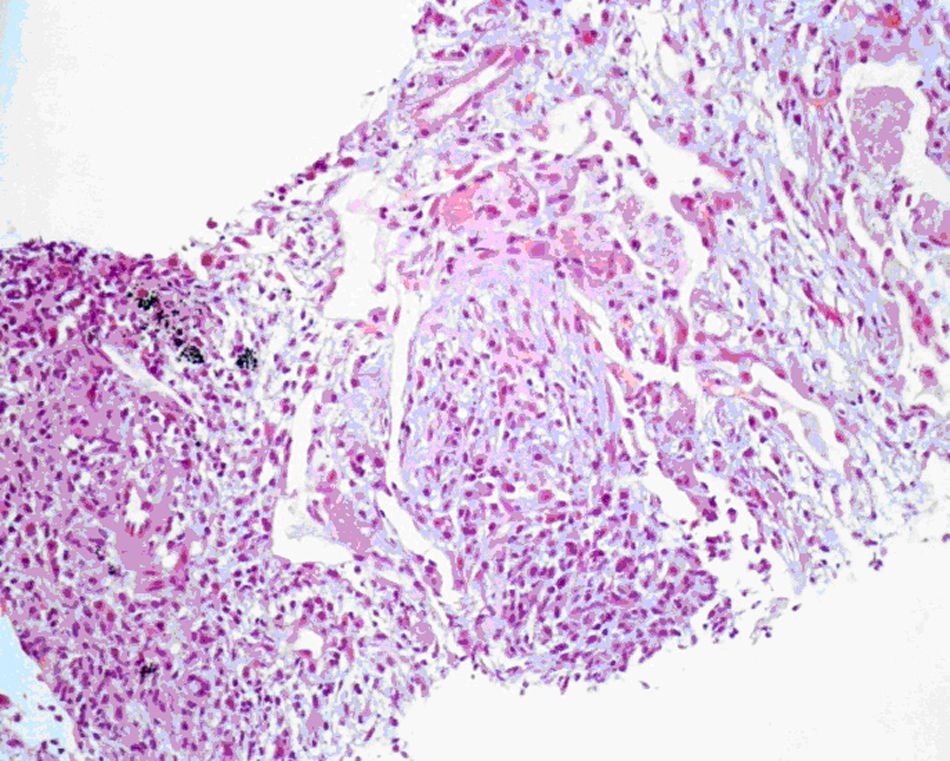
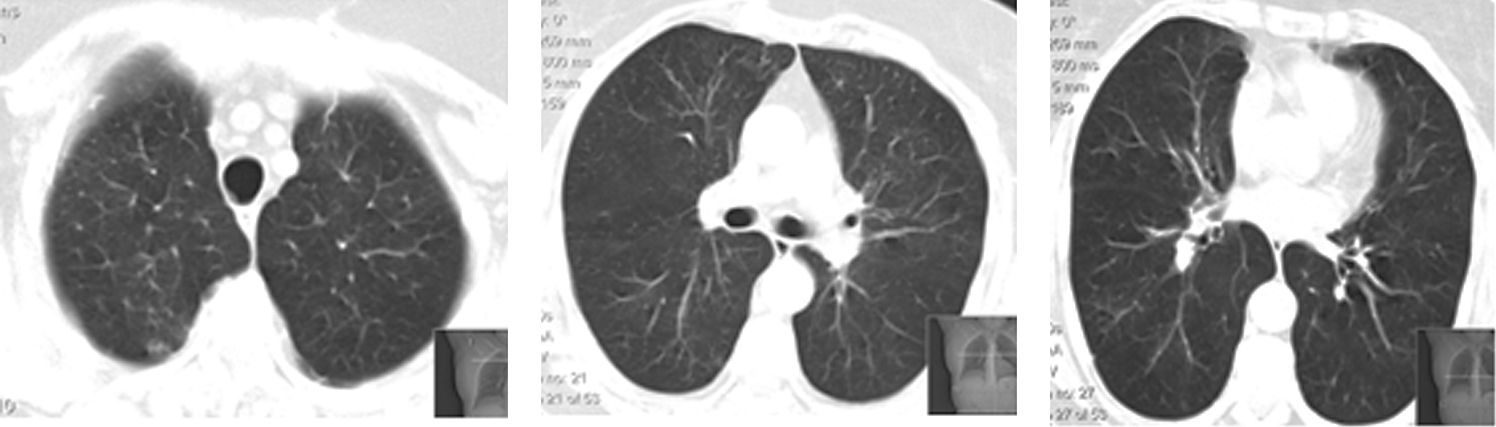
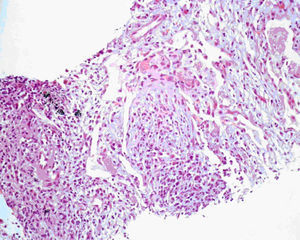
In the meanwhile, there was clinical deterioration with the patient presenting a moderate obstructive ventilatory syndrome (FEV1=53%) without bronchodilator reversibility, slight hipoxemia (Pa02=71.5mmHg) and significant desaturation in the six minute walk test (S02=90---95%, 500m). The patient was then referred for pulmonology consultation and a second transthoracic core biopsy was performed revealing features compatible with OP, namely chronic inflammation and intra-alveolar organizing fibromyxoid polyps (Fig. 2). The diagnosis of COP was established, since no aetiology related to OP was found. The patient started high dose of corticosteroid therapy (equivalent dose of prednisolone 1mg/Kg/day), but as there was no clinical improvement and tapering glucocorticoid therapy was not possible, four months later, azathioprine was added. The dose of azathioprine was not increased above 150mg/day, and the cytotoxic drug was stopped, due to persistent exacerbations and hospital admission. Following discharge, azithromycin, 500mg on alternate days, was started as an adjuvant to steroid therapy (0,75mg/kg/day). Clinical and functional improvement followed and HRCT showed clearing of lung infiltrates (Fig. 3). It was possible to withdraw steroid therapy within a year and there was no evidence of relapse in the six months of the follow up. Azithromycin was maintained, and at this point the frequency of administration was reduced (500mg, three times per week). No adverse side effects were observed.
Spontaneous remission of COP is rare and, therefore, the majority of symptomatic patients with lung infiltrates will need treatment.2,3 The prominent histological finding is OP with patchy involvement of the pulmonary parenchyma by fibromyxoid, polypoid plugs of granulation tissue, within the alveoli and occasionally the bronchioles.1–3 Intra-alveolar fibrosis of OP represents a unique model of inflammatory lung disease, since it is not associated with progressive irreversible fibrosis.2 Corticosteroids are, thus, the standard therapy, resulting in complete recovery in up to 80% of patients within a few weeks to three months.3 Relapses are common (13% to 58%), usually associated with tapering or withdrawal of corticosteroids. Even so, the prognosis of the disease is generally good.2,3 Immunosuppressive agents, such as cyclophosphamide and azatioprine, can be used in refractory COP, although data concerning the use of these drugs are scarce.3
The patient described presented further relapses with high dose of corticosteroid therapy and even with the addition of an immunosuppressant agent. Therefore, based on observational studies and case series reporting efficacy of chronic low-dose macrolide therapy with a 14 (erythromycin, clarithromycin) and 15 (azithromycin) member-ring in COP, azithromycin was started as an adjuvant to corticotherapy.4–9
Besides antibacterial actions, these macrolides seem to have immune modulating effects that appear to be the rationale for clinical benefit in several chronic inflammatory airway diseases.10,11
Much of the evidence that macrolides have immunomodulatory effects comes from Japan, where these antibiotics have proved effective in treating diffuse panbronchiolitis (evidence 1A).10–12 In cystic fibrosis, the most recent recommendations suggest that they should be reserved for those with chronic Pseudomonas aeruginosa infection when controlling symptoms and maintaining pulmonary function has been difficult (evidence 2A).11,13 The evidence for a role of macrolides in other inflammatory airway diseases, such as idiopathic bronchiectasis (evidence 2B), obliterative bronchiolitis (evidence 2B), chronic obstructive pulmonary disease (evidence 2B), COP (evidence 2C) and asthma (evidence 1B, against), is less clear.11Macrolides in COP have been described as an alternative therapy in patients with minimal symptoms and/or minimal physiologic impairment.4–6 In the majority of cases, patients were started on macrolides for suspected bacterial infection and subsequently received the diagnosis. Other patients either refused corticosteroid therapy or could not tolerate the side effects and were administered macrolides. The use of macrolides as adjuvant therapy in patients receiving steroids is also considered in the literature.7–9
In general, macrolides are assumed to reduce airway inflammation by several mechanisms such as modulation of host-pathogen interactions, signalling pathways, cytokine responses, oxidative stress, innate immunity and others, such as decrease in mucus secretion and methylprednisolone clearance.11,14–16 The mechanism of the action of macrolides in COP is not yet clear. However, it is known that some patients with the disease show a mixed BAL pattern with increased neutrophils, eosinophils and lymphocytes, with a decrease in CD4/CD8 ratio due to an increase in cytotoxic T-cells. Aoki and Kao16 demonstrated that erythromycin may exert anti-inflammatory effects on T-cells by inhibiting cytokine gene expression at the level of transcription activation, demonstrating that the beneficial effects of macrolides in COP may occur due to their immunosuppressive effect on polymorphonuclear cells and their products and also to their influence on T-cells.
Our patient did not however present lymphocytosis on BAL. In fact, the absence of BAL lymphocytosis, the presence of comorbilities and the late diagnosis, the three factors considered to have the worst prognosis, were recorded in this case. Nevertheless, there seemed to be a correlation between administration of the drug and the end of the recurrent episodes, with radiologic resolution and clinical and functional improvement.
Like the corticosteroids, macrolides were continued for a prolonged period of time, and were then empirically tapered after the withdrawal of the former. As with the majority of other case studies, we did not find any complications during long term therapy with macrolides.
Although this case may reinforce the role of macrolide anti-inflammatory properties in COP as corticosteroids adjuvant therapy, further studies are required in order to clarify potential benefits and possible side effects, in particular the most serious ones, related to antimicrobial resistance. In addition, information about which patients would be likely to respond, proper dosage and duration of macrolide therapy is needed.10,11
Please cite this article as: Vaz AP, et al. Azitromicina como terapêutica adjuvante na pneumonia organizativa criptogénica. Rev Port Pneumol. 2011; 17: 186–189.