Unsupervised PA interventions might have a role in the management of chronic obstructive pulmonary disease (COPD) but their effectiveness is largely unknown. Thus, we aimed to identify and synthesise data on the effects of unsupervised PA interventions in people with COPD.
Material and methodsDatabases were systematically searched in April 2020, with weekly updates until September 2021. Randomised controlled trials and quasi-experimental studies comparing unsupervised PA with usual care, were included. Primary outcomes were dyspnoea, exercise capacity and physical activity. The effect direction plot was performed to synthesise results. Meta-analysis with forest plots were conducted for the Chronic Respiratory Disease questionnaire – dyspnoea domain (CRQ-D), 6-minute walk distance (6MWD) and incremental shuttle walk distance (ISWD).
ResultsEleven studies with 900 participants with COPD (68±10 years; 58.8% male, FEV1 63.7±15.8% predicted) were included. All interventions were conducted at home, most with daily sessions, for 8-12 weeks. Walking was the most common component. The effect direction plot showed that unsupervised PA interventions improved emotional function, fatigue, health-related quality of life, muscle strength and symptoms of anxiety and depression. Meta-analysis showed statistical, but not clinical, significant improvements in dyspnoea (CRQ-D, MD=0.12, 95% CI 0.09-0.15) and exercise capacity, measured with 6MWD (MD=13.70, 95% CI 3.58-23.83). Statistical and clinical significant improvements were observed in exercise capacity, measured with ISWD (MD=58.59, 95% CI 5.79-111.39). None to minor adverse events and a high adherence rate were found.
ConclusionsUnsupervised PA interventions benefits dyspnoea and exercise capacity of people with COPD, are safe and present a high adherence rate. Unsupervised PA interventions should be considered for people with COPD who cannot or do not want to engage in supervised PA interventions or as a maintenance strategy of PA levels.
Chronic obstructive pulmonary disease (COPD) is a global public health concern.1 People with COPD present higher sedentary behaviour and lower levels of physical activity (PA) than their healthy peers.2 Physical inactivity has been associated with poor health outcomes (e.g., dyspnoea, exercise intolerance, reduced health-related quality of life [HRQoL]) in people with COPD,3,4 being an independent risk factor for hospitalisations due to acute exacerbations and early mortality.5,6 Therefore, improving PA levels in this population is imperative.1,7
Physical activity has well-established physiological, social and psychological benefits in people with COPD.1,8 Despite these unequivocal benefits,1 increasing PA levels in this population is often challenging.9 Barriers to engage in PA include low motivation,10,11 physical (e.g., symptoms-related) and psychological (e.g., fear) disease limitations,11 limited access to12 or lack of perceived benefit of PA interventions,13,14 time requirements and14 travel issues.13,14
Unsupervised PA may contribute to overcome some of these barriers as it: i) is low cost;15 ii) presents a broad application (e.g., specialised equipment is not required);15 and iii) can be undertaken in any environment and/or at any time, whatever suits the individuals best,16 hence may enhance adherence to PA in people with COPD. Nevertheless, unsupervised PA interventions are still underused in this population.15 One possible explanation could be the lack of synthesis of the most common unsupervised PA interventions and respective evidence.
Recently, a systematic literature review of unsupervised exercise-based interventions in this population was published.17 However, they focussed on exercise interventions, which is just a subset of PA.18 PA refers to all movement performed by an individual, which means other components besides exercise, such as everyday tasks, are included.18 In fact, people with COPD reduce their participation in PA and adopt a sedentary lifestyle to avoid exertional dyspnoea,11 leading to muscle deconditioning and accentuating exercise capacity impairment.19,20 Therefore, synthesising evidence of the benefits obtained with unsupervised PA interventions and also including activities integrated in individuals’ daily life may be highly meaningful for participants, and provide relevant information to healthcare professionals for the management of COPD, especially in limited resource settings.
Therefore, this systematic review aimed to identify which unsupervised PA interventions have been used for people with COPD and explore their effectiveness.
Material and methodsThis systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO – registration no. CRD42020162311) and follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines21 and the Synthesis Without Meta-analysis (SWiM)22 recommendations.
Eligibility criteriaStudies were included if: i) their sample was composed of adult (≥18 years) people with COPD in a stable phase of the disease (i.e., 4 weeks without hospital admissions or exacerbations, nor changes in medication, according to Global Initiative for Chronic Obstructive Lung Disease – GOLD report1); ii) included unsupervised PA interventions for people with COPD compared to usual care (i.e., had not received any PA intervention in the study period); iii) they were original randomised controlled trials (RCT) or quasi-experimental studies; iv) written in Portuguese, English, Spanish or French languages. Studies were excluded if they: i) involved proxy versions; ii) were qualitative studies; iii) included other treatments/activities as an intervention while performing PA; iv) included any directly supervised training (other than a single session), i.e., face-to-face or remote contact (e.g. video-conference); and, v) were performed in hospital-based settings.
For the purpose of this review, the following definition of PA was used: “any bodily movement produced by skeletal muscles that requires energy expenditure.”18 Unsupervised PA interventions were defined as any PA without any supervision, undertaken in any environment and/or at any time, which best suits the person.16 It could include a single supervised session to explain and/or demonstrate the activities; and, remote contact with healthcare professional using technologies, such as, telephone, mobile phone and/or tablet devices, to check patients’ health and monitor their evolution, without being used to interactively coach/instruct the patient (e.g., video-conference).
Information sourcesA systematic literature search was conducted in April 2020, on the following electronic databases: Cochrane Library, PubMed, Scopus, Web of science, EBSCOhost. Electronic search was supplemented by weekly automatic updates retrieved from the databases until September 2021, and hand-searches of references in key systematic reviews.17 The search strategy was performed by title, abstract, keywords/MESH term. The full search strategy is presented in Supplementary material - Appendix 1.
Study selectionAfter removing duplicates, two reviewers (CP and VR) independently screened the potential studies (title and abstract), according to the eligibility criteria. The full-text of each potentially relevant study was then independently screened by the same reviewers to decide on its inclusion. Discrepancies were solved by consensus, and if agreement could not be reached, the other authors’ opinion was obtained. Primary outcomes were dyspnoea, exercise capacity and PA. Secondary outcomes included body composition, emotional function, fatigue, health behaviours, healthcare utilisation, HRQoL, mastery, muscle strength, self-efficacy, symptoms of anxiety and depression, adverse events and, dropouts and adherence to interventions.
Data extractionOne reviewer independently extracted the data from included studies, and the other authors checked the accuracy and completeness of information. Data extraction was performed using a pre-developed and structured table-format covering the following topics: characteristics of the study (first author, year of publication, country and study design); setting (i.e., home-based); population (number of participants, sex, age, forced expiratory volume in one second percentage of predicted [FEV1pp], severity of airway limitation [GOLD grades 1-4]1 and comorbidities [type and severity, classified with Charlson Comorbidity Index-CCI]; intervention (type, frequency and duration); outcome and outcome measures; and, results obtained in each outcome measure. Studies with multiple publications were identified to avoid duplicate reports (e.g., number of participants). Corresponding authors of the included studies were contacted via e-mail to request additional data (i.e., means and SD), whenever needed.
Quality assessmentTwo reviewers independently assessed the methodological quality of each study using the Quality Assessment Tool for Quantitative Studies, developed by the Effective Public Health Practice Project, Canada.23 This tool is comprised of six domains of methodological quality: 1) selection bias; 2) study design; 3) confounders; 4) blinding; 5) data collection methods; and, 6) withdrawals and dropouts.23 Each domain is rated as “strong”, “moderate” or “weak”, according to a standardized guide.24 The overall rating of each study is determined by the total number of “weak” scores, i.e., if the study presented: i) no weak scores, it was rated as strong quality; ii) one weak rating - moderate quality; and, iii) two or more weak ratings - weak quality.23
Data analysis and synthesisInter-rater agreement analysis was assessed using Cohen's kappa to explore the consistency of the quality assessment performed by the two reviewers. The Cohen's kappa ranges from 0 to 1 and agreement was interpreted as: slight (⩽0.2), fair (0.21–0.4), moderate (0.41–0.6), substantial (0.61–0.8), or almost perfect (≥0.81).25
Studies were grouped according to the outcome measures reported. An effect direction plot was computed to deal with the diversity of outcome measures used in the included studies, following the SWiM recommendations.26 This plot considers the study design, effect estimates of each outcome (represented with arrows, i.e., upward arrow ▲= positive health impact, downward arrow ▼= negative health impact, sideways arrow ◄►= no change/mixed effects/conflicting findings), sample size and studies quality (using a traffic light system, i.e., green for studies of high quality, amber for moderate and red for weak quality of evidence).26 The effect estimates were analysed with the Cohen's d effect sizes (ES) based on the Pre/Post means and SD, according to the formula of Morris.27 The ES were interpreted as very small (≥0.01), small (≥0.20), medium (≥0.50), large (≥0.80), very large (≥1.20) and huge (≥2.0).28,29 Results were analysed by counting the effect direction and interpreted using the proportion of effects favouring the intervention 30 Proportions higher than 50% were considered as an improvement in the respective outcome measure.30
Meta-analysis, with forest plots, only included studies reporting the mean changes between the experimental (EG) and control (CG) groups and the respective SD or data allowing the calculation of these estimates. Between-study heterogeneity was quantified using I-squared (I2) statistic. Statistical homogeneity was defined as ≤40%.30
Some data transformation occurred to compute ES. Data presented as 95% of confidence intervals (95% CI) 31 were transformed into SD, using the formula: SD=n*(upperlimit−lowerlimit)/3.92, where n is the sample size.30 Additionally, data presented as median and interquartile range (IQR) 31 were converted into mean and SD using the summary table proposed by Wan and colleagues.30,32
Data analysis were performed using IBM SPSS 24.0 (IBM, Armonk, New York, USA) and RStudio, V1.2.5033 (RStudio, Inc; Boston, MA, USA).
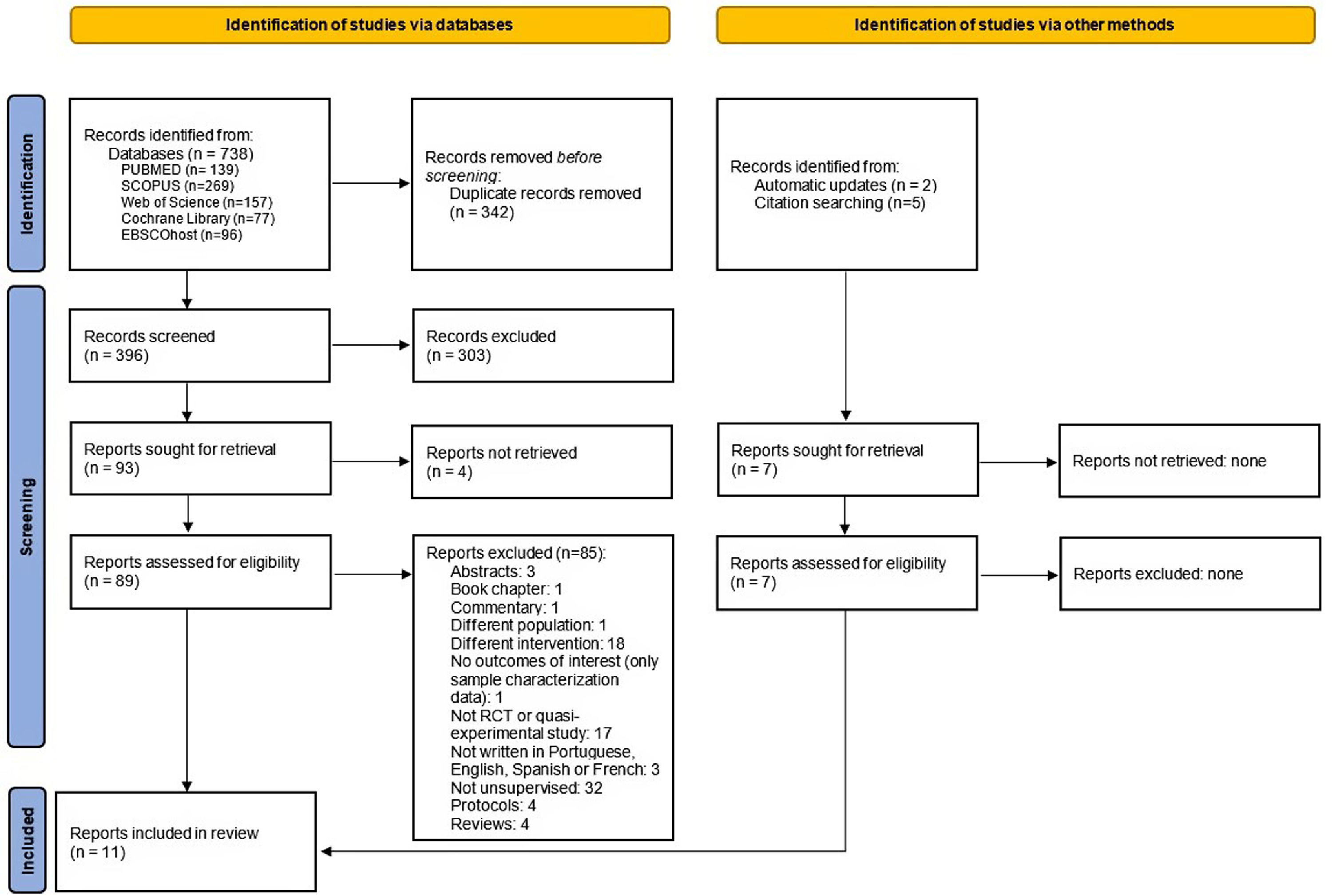
ResultsStudy selectionThe literature search provided 738 studies. After duplicates removed, 396 records were screened and 303 were excluded. The full-text of 93 articles was assessed and four studies were included. Seven additional studies were identified and retrieved, two from the databases weekly automatic updates and five from the reference list of a key systematic review 17 (Fig. 1). A total of 11 articles were included.
Quality assessmentFour studies were rated as strong 33-36 (36%), three 31,37,38 (28%) as moderate and four 39-42 (36%) as weak quality. Inter-rater agreement was substantial (Cohen's Kappa=0.72; 95% CI=0.37-1.07; p = 0.003; percentage of agreement= 82%). Quality assessment details can be found in Supplementary material (Table S1).
Studies characteristicsIncluded studies were published between 197742 and 202031, and were mostly conducted in the United States of America,33,34 Australia,31,39 United Kingdom 36,38 and Taiwan.35,41 A total of 900 participants with COPD, 446 in the EG and 454 in the CG, were included. Sample sizes ranged between 2038 to 305 participants.33,34 Participants were on average 68±10 years old (n = 6), 541 (59%; n = 9) were male, presented a mean FEV1 of 63.7±15.8 %predicted (n = 3) and the majority had moderate to severe (GOLD grades 2-3) grades of the disease (n = 4). Comorbidities reported (n = 3) included: cardiovascular disease (hypertension, heart failure, myocardial infarction, and peripheral vascular diseases), metabolic syndrome (e.g., diabetes), depression, musculoskeletal disease (e.g., arthritis) and ulcer disease.33,34,36Table 1 presents detail characteristics of the included articles.
Design and effects of unsupervised physical activity interventions for people with chronic obstructive pulmonary disease (n = 11).
5STS, five times sit-to-stand test; 6MWD, 6-minute walking distance; 6MWT, 6-minute walk test; 12MWD, 12-minute walking distance; 95% CI, 95% of confidence intervals; %, percentage; ADLs, activities of daily living; BCKQ, Bristol COPD Knowledge Questionnaire; bpm, beats per minute; CAT, COPD assessment test; CCI, Charlson Comorbidity Index; CG, control group; cpm, cycles per minute; CRQ, Chronic Respiratory Questionnaire; CRQ-D, CRQ – dyspnoea domain; EE, energy expenditure; EG, experimental group; ES, effect size; ESWT, endurance shuttle walk test; FEV1pp, forced expiratory volume in 1 second – percentage predicted; HADS, Hospital Anxiety and Depression Scale; HADS-A, HADS – anxiety; HADS-D, HADS – depression; HRex, heart rate during the greatest work load that a subject could maintain for 1 minute; HRQoL, health-related quality of life; HRst, heart rate during the greatest work load that was common to both the initial and follow-up exercise in any one subject; ISWD, incremental shuttle walk distance; kg, kilograms; l/min, litres per minute; m, meters; MBS, modified Borg scale; min/day; minutes per day; mm, millimetres; mmol/min, millimole per minute; mMRC, modified British Medical Research Council; MVPA, moderate-vigorous physical activity; NA, Not applicable; ND, Not described; Nm, Newton meters; no, number; PA, physical activity; PR, pulmonary rehabilitation; PRAISE, Pulmonary Rehabilitation Adapted Index of Self-Efficacy; PT, peak torque; pts, points; PT/BW, peak torque/body weight; pts, points; RAPA, Rapid Assessment of Physical Activity questionnaire; Rex, respiratory exchange ratio during the greatest work load that a subject could maintain for 1 minute; RCT, randomised controlled trial; s, seconds; SF-36, 36-item short form survey; SGRQ, St. George's respiratory questionnaire; TDI, Transition Dyspnea Index; UK, United Kingdom; W, Watts; WLex, work load during the greatest work load that a subject could maintain for 1 minute; VEex, minute ventilation during the greatest work load that a subject could maintain for 1 minute; VO2ex, oxygen uptake during the greatest work load that a subject could maintain for 1 minute.
Interventions lasted from 6 36,38 to 66 weeks,33,34 being 8-12 weeks 31,35,37,39-42 the most common range duration, and were performed 3 days/week,37 4 days/week 38 or daily. 31,33-36,39-42 All interventions were designed by health professionals (i.e., general practioners,35,42 nurses35,37,39-41, physiotherapists31,36,38 and health coachs33,34) and performed at home.31,33-42
Interventions were single-component in seven 33-35,37,39,41,42 and multi-component in four 31,36,38,40 studies. Aerobic exercise 31,35,36,38,39,41,42 (e.g., walking) and muscle strength 31,36-38,40 were the main training components. Two studies 33,34 focused on promotion of lifestyle PA (i.e., promotion of activities of daily living). Interventions also included diaries;31,41,42 action plans;39,41 information about healthy behaviours;39 phone calls to support the intervention, promote healthy behaviours or deliver self-management training;31,33,34,36,39,40 distribution of handbook/manual;33,34,41 workbook activities;33,34 reading assignments;33,34 and nutritional and psychosocial support.40 More details are presented in Table 1.
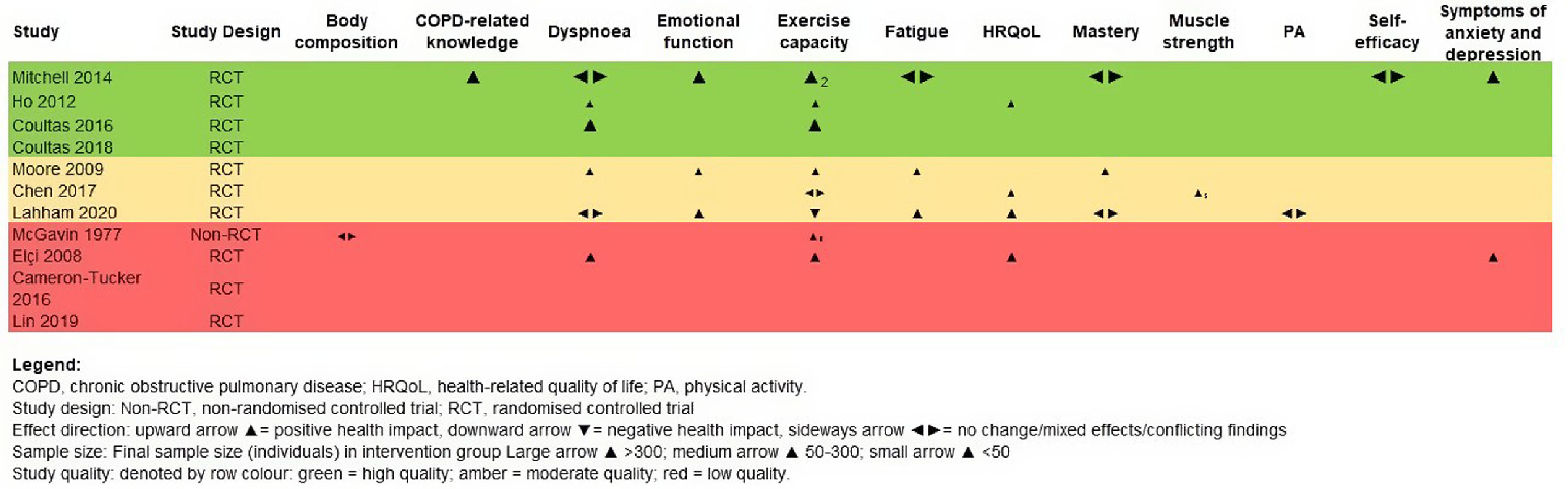
Effectiveness of unsupervised PA interventionsA total of 14 outcomes, evaluated by 44 different measurement tools were found in the included studies (Table S1, Figs. 2 and 3).
Dyspnoea was measured in seven studies, with the modified Borg scale (MBS)– dyspnoea,35 the Chronic Respiratory Questionnaire - dyspnoea domain (CRQ-D) 31,34,36,38 and the modified British Medical Research Council (mMRC).31,41
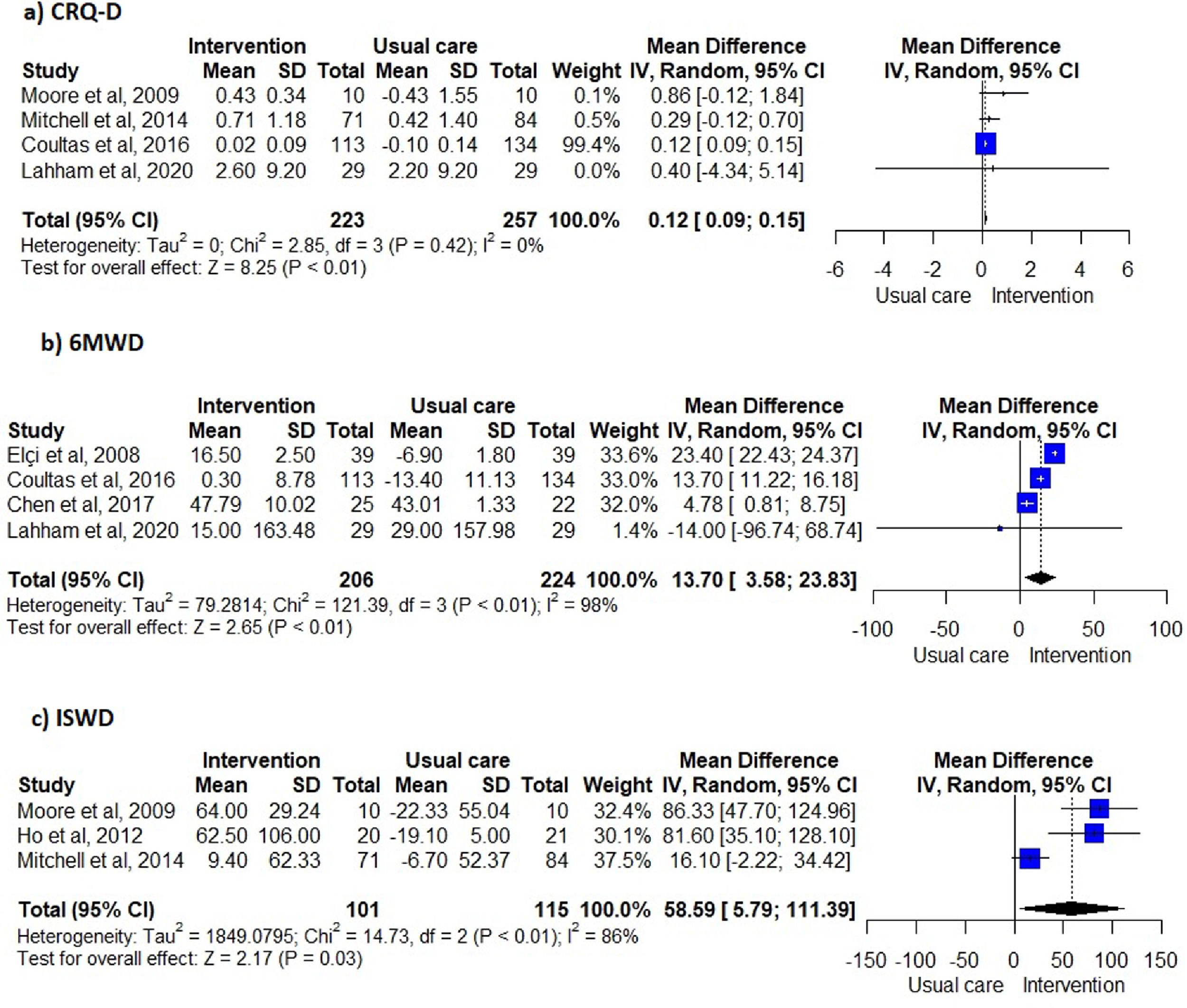
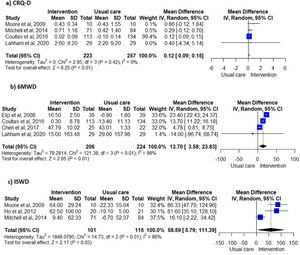
Unsupervised PA interventions had a positive effect on dyspnoea, with four 34,35,38,40 of six studies 31,34-36,38,40 favouring the EG (67%, 95% CI 22-96%) (Fig. 2). Meta-analysis of the CRQ-D34,35,38,40 showed this significant improvement (MD=0.12, 95% CI 0.09-0.15). Statistical heterogeneity was not apparent (I2=0%) however, the intervention effect was heavily weighted towards one trial34 (Fig. 3).
Exercise capacityExercise capacity was measured in nine studies.31,34-40,42 Most common measures used were the 6-minute walk distance (6MWD)31,34,37,39,40 and the incremental shuttle walk distance (ISWD).35,36,38 The 12-minute walk distance,42 the heart rate,42 the respiratory exchange ratio,42 the minute ventilation,42 the oxygen uptake,42 the work load,42 the stride42 and the endurance shuttle walk test36 were also reported. A positive effect on exercise capacity was observed, with six34-36,38,40,42 of the eight studies31,34-38,40,42 favouring the EG (75%, 95% CI 35-97%) (Fig. 2). These positive effects were also observed in meta-analysis of the 6MWD31,34,37,40 (MD=13.70, 95% CI 3.58-23.83) and ISWD35,36,38 (MD=58.59, 95% CI 5.79-111.39). However, a substantial heterogeneity was observed in both meta-analysis (I2=98%, p < 0.01; I2=86%, p < 0.01; respectively) (Fig. 3).
Physical activityPhysical activity was assessed in three studies,31,33,39 using METs/day,31 moderate-vigorous PA (MVPA) bouts and time,31 Rapid Assessment of Physical Activity questionnaire,33 “SNAPPS” (Smoking, Nutrition, Alcohol, Physical activity, Psychosocial wellbeing and symptom management) snapshot questionnaire self-reported walking,39 sedentary bouts and time,31 steps/day,31 time spent in MVPA31 and in sedentary bouts31 and total energy expenditure.31 The direction of effect was only evaluated in one study,31 however a consistent direction of the effect was not determined.
Secondary outcomesBody compositionBody composition was assessed in one study using weight and no differences were observed between groups.42
Emotional functionEmotional function was assessed with the CRQ-emotional function domain, in three studies31,36,38 and positive effects were observed, favouring the EG (100%, 95% CI 29-100%).
FatigueFatigue was assessed with the CRQ-fatigue domain in three studies,31,36,38 and a positive effect was found in two31,38 of these studies, favouring the EG (67%, 95% CI 9-99%) (Fig. 2).
Health behavioursHealth behaviours were evaluated using the SNAPPS snapshot questionnaire, total score and per domains, in one study.39 Direction of the effect was not possible to be determined and no significant differences were observed between groups.39
Healthcare utilisationHealthcare utilisation was assessed in two studies.33,35 One used the number of emergency visits, hospitalisations, unscheduled clinic visits and length of hospitalisations35 and the other the lung-related health care utilisation.33 No differences were observed between groups, although lung-related health care utilisation was lower in the EG after the intervention (risk ratio=0.68, 95% CI 0.47-1 and rate ratio=0.64, 95% CI 0.42-0.99).33
Health-related quality of lifeHealth-related quality of life was evaluated in six studies,31,35,37,39-41 using the 36-item short form survey,40 the COPD assessment test,37,39,41 the CRQ total score31 and the St. George's Respiratory Questionnaire.35,40 Direction of effect was only possible to be determined in four studies.31,35,37,40 Unsupervised PA interventions had a positive effect on HRQoL, favouring the EG (100%, 95% CI 40-100%) (Fig. 2).
MasteryMastery was assessed with the CRQ-mastery domain, in three studies31,36,38 and no effects were observed, with only one study 38 favouring the EG (33%, 95% IC 1-96%) (Fig. 2).
Muscle strengthLower-limb muscle strength was evaluated in one study,37 with the five times sit-to-stand test, isokinetic and isometric peak torque and adjusted for body weight. Improvements were observed in all outcome measures (ES= -0.36 to 0.26) for the EG after the intervention.37
Self-efficacySelf-efficacy was assessed in one study36 with the Pulmonary Rehabilitation Adapted Index of Self-Efficacy and no differences were observed between groups.
Symptoms of anxiety and depressionSymptoms of anxiety and depression were measured in three studies36,40,41 with the Hospital Anxiety and Depression Scale. Positive effects were observed, with two36,40 studies favouring the EG (100%, 95% IC 16-100%) (Fig. 2).
Adverse eventsFour studies33,34,37,41 explored the adverse events of unsupervised PA interventions. Two33,34 of these studies found that 63% (n = 192) of participants had no adverse events and 37% (n = 106) had, at least, one adverse event. The most common adverse event was acute exacerbation of COPD, with a twice higher prevalence in the CG (15%, n = 47) than in the EG (9%, n = 28) (p < 0.01).33,34
Dropouts and adherence to interventionsNine studies33-39,41,42 reported dropouts, ranging between 7.1%41 to 38.5%.39 Reasons to dropout included: abrupt dizziness,37 acute exacerbation of COPD,35,38 cataract surgery,37 comorbidities,36 death,42 failure to keep appointments,39 intercurrent depressive illness,42 knee pain,38 lack of enthusiasm,42 lost to follow-up,36,38,41 non-COPD related hospital admission,35,37 poor health,39,41 social reasons,36 programme was too easy or not so serious,36,37 time constraints,35,39 travel issues,37,39 too busy to participate 41 and work commitement.36
Only four studies reported adherence to the intervention,31,35,37,39 which varied between limited39 to 93%.31
DiscussionThis systematic review provided an overview of the unsupervised PA interventions implemented in people with COPD and showed that these interventions are effective in improving dyspnoea and exercise capacity.
Unsupervised PA interventions were conducted at home,31,33-42 in most cases lasted 8-12 weeks31,35,37,39-42 and were performed daily.31,33-36,39,41,42 Aerobic training was the most common component,31,35,36,38,39,41,42 namely, walking, however strength training 31,36-38,40 was also included and done in isolation33-35,37,39,41,42 or with others.31,36,38,40 These findings are of special importance, since people with COPD spend most of their day in a sedentary behaviour and at home.2,43 Therefore, conducting these interventions in patients’ home-environment, integrated into their daily routines (e.g., aerobic training through walking31,35,39 or home stairs31) and using everyday resources (e.g., water bottles as weights31), may be a person-centred and feasible approach to increase participation in PA for people with COPD. Therefore, such interventions should be considered for those people with COPD who cannot or do not want to be involved in supervised PA interventions, either by limited access or disease restrictions, or as a strategy for maintaining PA levels (e.g., after pulmonary rehabilitation), with regular assessments and/or phone calls for monitoring the individuals’ progress.44
Furthermore, these interventions were effective in improving dyspnoea and exercise capacity in people with COPD. Nevertheless, some caution is needed when interpreting the effects of unsupervised PA interventions in dyspnoea for several reasons. First, the meta-analysis for the CRQ-D was greatly weighted by one large study,34 with other studies showing no effects. This might have led to an underestimation of the effect. Also, the observed improvement (0.12 points) in the CRQ-D was statistically significant but not clinically relevant, based on the minimal clinically important difference (MCID) of 0.5 points of CRQ-D.45 Therefore, further studies with higher sample sizes assessing the effects of unsupervised PA interventions in dyspnea should be conducted, since this outcome is a cardinal symptom for people with COPD.1 In terms of exercise capacity, unsupervised PA interventions lead to statistical improvements under the MCID (25m46) in the 6MWT, but statistical and meaningful improvements above the MCID (47.5m46) in the ISWT. Heterogeneity of the interventions might explain this finding. Interventions included in the 6MWD meta-analysis were heterogeneous, whilst all the interventions included in the ISWD meta-analysis were walking-based. Integrating a walking component into the unsupervised PA interventions seems therefore important to improve exercise capacity clinically.
Similar results, for dyspnoea and exercise capacity (measured with 6MWD), were found recently in a systematic review of unsupervised exercise-based interventions in this population.17 This is of special importance for clinical practice and research communities, which have been increasingly focusing on the promotion of PA and can now see benefits in these outcomes also obtained with unsupervised PA interventions integrating activities of individuals’ daily life.8,47
We were unable to draw conclusions using the effect direction plot for PA. Indeed, it is surprising that PA, a strong predictor of COPD progression,48 was just assessed in three studies. Thus, studies assessing the effects of unsupervised PA interventions in PA levels of individuals with COPD are urgently needed.
Our findings also showed that unsupervised PA interventions were effective in improving emotional function, fatigue, HRQoL, lung-related healthcare utilisation, muscle strength, self-efficacy and symptoms of anxiety and depression. Prior studies have shown that these parameters play a role in the disease management and progression1,49-53 however, evidence is still scarce. Given the social, economic and health burden of COPD worldwide,1 further research focusing on the effects of unsupervised PA interventions on these outcomes is needed.
Overall, unsupervised PA interventions were shown to be safe, with no or minor adverse events being reported. Most of the included studies reported a high adherence to unsupervised PA interventions. Compared with supervised PA interventions, unsupervised PA interventions showed a higher rate of adherence.54 These interventions are adapted to each person´s context and needs, are low cost and have broad applicability, being easy to perform at home, which might explain the high levels of adherence.15 Future research should explore important variables such as GOLD grades and groups and its influence on the results obtained, as well as the long-term effects of such interventions.
LimitationsThis systematic review has several limitations that need to be acknowledged. Firstly, the small number of existing studies; their large diversity of designs, outcomes and outcome measures; lack of consensus on the definition of unsupervised PA; and, the high heterogeneity observed in the meta-analysis, limited our conclusions. Nevertheless, a synthesis of the results, using the effect direction plot was computed which provided a thorough synthesis of data. Secondly, the imbalance between participants, i.e., more males than females and moderate to severe participants, limited the generalisation of results. Further studies including more people with COPD in mild and very severe grades and females should be conducted. Thirdly, our search was limited to studies published in English, Portuguese, Spanish or French included in databases. Additional studies may exist in the unpublished grey literature and may have been missed. A thorough search in different databases and scanning the references of key articles and systematic reviews were however conducted to minimise this limitation. Finally, approximately one third of the included studies were of low quality, nevertheless most studies presented moderate to high quality.
ConclusionsThis systematic review showed that unsupervised PA interventions improved dyspnoea (statistically but not clinically) and exercise capacity in people with COPD. Overall, these interventions seem to be safe and present a high adherence rate. The inclusion of a walking component for 8-12 weeks in the unsupervised PA interventions is recommended to optimise results. Unsupervised PA interventions should be considered for people with COPD who cannot or do not want to engage in supervised PA interventions or as a maintenance strategy of PA levels. Future studies with robust methodologies should now be conducted to strengthen these promising results with potential to optimise COPD management.
Funding sourcesThis project is funded by Programa Operacional de Competitividade e Internacionalização – POCI, through Fundo Europeu de Desenvolvimento Regional - FEDER (POCI-01-0145-FEDER-028806), by Fundação para a Ciência e a Tecnologia through the European Social Fund and Programa Operacional Regional do Centro (PTDC/SAU-SER/28806/2017, PhD grant SFRH/BD/148741/2019) and under the project UIDB/04501/2020.
Disclosure of interestThe authors have no conflicts of interest to declare.
PROSPERO registration number: CRD42020162311.