To the Editor
The use of superlatives — exaggerated language meant to influence perceptions — is a technique used by journalists to attract and persuade readers. However, superlatives used to describe medical therapies have the potential to misrepresent their efficacy. Previous studies have found the use of superlatives in news articles related to cancer therapies.1 This type of sensational reporting is problematic, because consumers use online search engines to browse treatment recommendations rather than reference evidence-based medical literature, because of feasibility. While ways to evaluate the quality of news articles exist, such as the Health on the Net Foundation (HONcode),2 treatment recommendations found through news outlets may spread medical misinformation, especially if not HONcode certified.3 Here, we sought to quantify the use of superlatives in news stories related to asthma treatments.
Our methodology was adapted from Abola and Prasad.1 On October 15, 2021, four investigators (R.M., C.H., C.S., H.M.) used the advanced search feature on Google News (https://news.google.com) to search “asthma treatment” with 10 separate superlative terms (Table 1) for articles published between October 15, 2020 and October 15, 2021. These terms were initially selected by Abola and Prasad.1 The articles were screened by two authors (C.S. and H.M) in double-blinded, duplicate fashion, followed by reconciliation and third party (R.M.) adjudication as needed. The following information was extracted if the superlative in the article was in reference to an asthma treatment: URL, website name, superlative(s), drug(s) or treatment(s), Food and Drug Administration (FDA) approval status, clinical data and support presence, author background, mention of COVID-19 or coronavirus and whether its mention was tangential to the focus of the article, and presence of HONcode certification listed on the website in which the article was found.
Characteristics of superlatives in news articles pertaining to asthma treatments.
*The superlatives marvel, home run, and revolutionary were not found in the news articles which met inclusion criteria
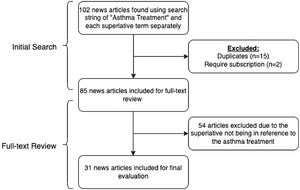
The initial search returned 102 news articles. Following removal of duplicates and subscription articles, 85 articles were selected for full-text review. Thirty-one news articles met inclusion criteria (Fig. 1). The characteristics of superlatives in reference to asthma treatments are displayed in Table 1. There were 49 instances of a superlative referencing asthma treatments, with “breakthrough” as the most common superlative used (n=23). The superlatives “marvel”, “home run”, and “revolutionary” were not found in articles meeting inclusion criteria. The two most common asthma treatments with superlatives in reference were budesonide (13/31, 41.9%) and tezepelumab (12/31, 38.7%). Nearly half (15/31, 48.4%) of the articles mentioned COVID-19 or coronavirus. Only 9.7% (3/31) of the articles were written by a medical writer, with 87.1% (27/31) of articles written by a journalist/editor, and one with author information not listed. Nine of the treatments were FDA approved, and four were in clinical trials. None of the included websites were HONcode certified.
A large percentage of articles in our study used superlatives to describe tezepelumab, a monoclonal antibody still in clinical trials. Interestingly, the article using the most superlatives referencing an asthma treatment pertained to preliminary stages of pharmacological development. Exaggerated language in reference to asthma treatments lacking FDA approval may mislead reader perception of therapeutic efficacy. The mention of COVID-19 or coronavirus in nearly half of the articles intending to cover asthma treatments may indicate a focus misalignment. The addition of the pandemic in these articles is likely meant to attract readers (“clickbait”),4 and may complicate their understanding of which disease the treatment targets. Further, none of the websites were HONcode certified, questioning the reliability of the articles’ health information due to lack of regulation.2
The pronounced lack of regulation across websites the news articles were found in may explain the presence of superlatives used toward non-FDA approved treatments, as well as the mention of COVID-19 or coronavirus, as each of these can potentially complicate readers’ understanding of asthma treatments instead of enhancing it. Overall, our findings indicate many news articles covering asthma treatments use exaggerated language and reference COVID-19, potentially complicating the publics’ understanding and trust of important medical information.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations: Health on the Net Foundation (HONcode), Food and Drug Administration (FDA)
Author Number Explanation: The large amount of time required to execute the study and write the manuscript, as well as the need for guidance by an experienced researcher, necessitated contributions by five authors to this study.