Current guidelines recommend the use of inhaled corticosteroids (ICS) for patients with moderate to severe persistent asthma treatment maintenance.1 However, the use of high doses for long periods potentially increase systemic adverse events.2,3 A disconnection between clinician estimates of ICS side effects and the prevalence reported by patients has previously been reported.4 Furthermore, there is evidence that many asthmatic patients prefer not to discuss or spontaneously report their concerns regarding ICS adverse events with their health care professionals.5,6 The self-reported questionnaires provide an efficient method of accessing information about adverse events.7,8 We describe systemic adverse events associated with the use of ICS in patients with moderate to severe asthma using this technique.
Subjects included were 18 or older, had moderate or severe persistent asthma,1 had been regularly using ICS for 6 or more months between June of 2010 and February of 2011 and presented at the Pharmaceutical Assistance Service of the Pneumology Reference Outpatient Clinic of the Federal University of Bahia, in Salvador, Bahia. Patients taking oral, parenteral, ocular or topical corticosteroids within the previous three months were excluded.
A pilot study assessing questionnaire structure, content and clarity generated data for an expert team who reviewed results and suggested changes. The self-report questionnaire, covered the previous 14 days using a 4-point Likert scale (0=never; 1=occasionally; 2=most days; 3=daily) and captured patient's perceptions regarding the ICS systemic adverse events (dry skin, swollen face, easy bruising, mood swings, night sweating, brittle breaking nails, hair loss and affected vision). The total score ranged from 0 (no perception of events) to 24 (maximum), this score was standardized to score from 0 (no events) to 100 (worst).
The difference in the intensity of systemic adverse events between the two dosage groups in relation to the total score (accumulated) was analyzed using t-test. The correlation between duration of ICS use and the number of systemic adverse events was assessed by using the Spearman Rho correlation coefficient.
Of the 65 patients who were evaluated, 54 (83.1%) were female, with an average age of 49.7 [SD=12.2] years. Of the total, 29 (44.6%) patients were taking high doses of ICS (budesonide>800mcg/day), where the median daily dose of ICS was 800mcg. The average treatment duration was 38.2 [30.7] months. Sixty (92.3%) patients reported at least one systemic adverse event, and 31 (47.7%) patients reported daily symptoms. A total of 213 events were reported with a median of 3.0 per patient (Table 1).
Frequency of systemic adverse events reported by 65 moderate or severe asthmatics of Pneumology Reference Outpatient Clinic of the Professor Edgard Santos University Hospital Complex of the Federal University of Bahia between June 2010 and February 2011.
| Adverse event | Frequency | Total n (%) | ||
|---|---|---|---|---|
| Occasionally n (%) | Most of the days n (%) | Daily n (%) | ||
| Night sweating | 4 (6.2) | 7 (10.8) | 19 (29.2) | 30 (46.2) |
| Brittle breaking nails | 6 (9.2) | 10 (15.4) | 13 (20) | 29 (44.6) |
| Vision affected | 8 (12.3) | 9 (13.8) | 12 (18.5) | 29 (44.6) |
| Dry skin | 8 (12.3) | 9 (13.8) | 15 (23.1) | 32 (49.2) |
| Mood swings | 9 (13.8) | 12 (18.5) | 12 (18.5) | 33 (50.8) |
| Hair loss | 3 (4.6) | 8 (12.3) | 13 (20) | 24 (36.9) |
| Bruising easily | 7 (10.8) | 6 (9.2) | 7 (10.8) | 20 (30.8) |
| Swollen face | 7 (10.8) | 4 (6.2) | 5 (7.7) | 16 (24.6) |
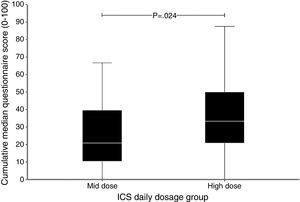
All patients were taking ICS plus long-acting β2-agonist (87.7% formoterol plus budesonide, 12.3% salmeterol plus fluticasone). Thirty-six (55.4%) patients received treatment consistent with moderate asthma and 29 (44.6%) received high-intensity treatment, consistent with severe asthma. Demographic characteristics were similar between the two groups. The prevalence of vision disturbances and dry skin was greater in the high-intensity treatment group (p<0.05) suggesting a causal relationship. Asthma severity (r=0.274; p=0.027) and ICS use duration (r=0.361; p=0.003) were correlated with the number of systemic adverse events. Furthermore, patients who used ICS for longer periods reported more face swelling (p=0.04) and dry skin (p=0.002). Patients taking high-intensity treatment presented a greater number of systemic adverse events than those taking lower doses (3.9±15.3 and 2.8±14.9, respectively; p=0.028). The total score of the adverse event assessment questionnaire also showed a statistically significant increase in the intensity of the systemic events, observed with the increase of the daily dose of ICS (Fig. 1). Our findings of elevated perception of systemic events induced by ICS corroborate the data of previous studies that used self-report questionnaires.4,7
Although the small sample size could be limitation, the importance of the study is to raise the possible dose-related association with ICS. We cannot rule out the possibility of our findings be biased in terms of older age and female gender. However, we identified the fact that the prevalence of vision disturbances and dry skin, events associated with advanced age, was greater in the high-intensity treatment group (p<0.05) suggesting a dose dependent association that could not be explained by age or gender because these two features were similar between the groups. Although the questionnaire administered in this study was designed to explore the occurrence of ICS systemic events, the possibility that, for some, the events may be related to characteristics of patients (such as age, sex and comorbid features) and others concomitant drugs cannot be totally excluded.
This study shows that individuals with moderate to severe asthma, assessed in real life, have a high perception of systemic adverse events, including mood swings and dry skin. Strategies to detect and manage ICS adverse events in clinical practice may be useful in order to improve the results of pharmacotherapy for asthma.
Conflicts of interestThe authors have no conflicts of interest to declare.