Sclerosing pneumocytoma (SP) usually discovered incidentally is a benign tumor and has a favorable prognosis with simple surgical resection. It is very important that accurate intraoperative diagnosis helps to avoid unnecessary lobectomy and lymph node dissection.1 Nevertheless, intraoperative consultaion of SP may be extremely difficult because it can be confused with malignancy, posing an intraoperative pitfall for the pathologist.2 Herein, we reported a case of SP with rosette structure mimicking carcinoid.
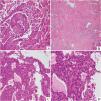
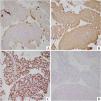
A 20-year-old female presented to the respiratory clinic of our hospital with cough and sputum for 5 days. Thoracic computed tomography (CT) revealed a solitary, well-circumscribed, regular solid nodule located at the lower lobe of the right lung, measuring approximately 24 mm × 21 mm (Fig. 1A). The patient underwent a resection of the nodule. In intraoperative consultation, the tumor was extremely well-circumscribed, unencapsulated, easily extracted, solid, and heterogeneous in texture. The tumor cells were round or polygonal without obvious atypia and mainly arranged in sheets (Fig. 1B), forming a solid growth pattern with a typical rosette structure, which was reminiscent of carcinoid tumor (Fig. 1C, D). An intraoperative consultation was rendered as carcinoid. Permanent sections analysis demonstrated similar findings to the frozen section material. However, the classical papillary pattern composed of SP with surface cuboidal cells lining the papillae and round or polygonal stromal cells within the papillae were observed (Fig. 2A). In these papillary areas, the immunohistochemistry (IHC) study for TTF-1 demonstrated strong nuclear staining for both surface cuboidal cells and round or polygonal stromal cells. In addition, cytokeratin AE1/AE3 and Napsin A showed immunoreactivity in the surface cuboidal cells with nonreactivity in the round or polygonal stromal cells. These papillary regions merged with a sclerotic pattern consisting of sheets of collagen with variable amounts of round cells (Fig. 2B). Moreover, rosette structures were still found in the postoperative permanent sections (Fig. 2C). The tumor cells in these rosette areas were consistent in size and shape without obvious atypia (Fig. 2D). In these rosette structures areas, surface cells were positive for AE1/AE3, similar to papillary areas (Fig. 3A). Furthermore, all tumor cells in these regions were positive for Vimentin and TTF-1 (Fig. 3B and C), whereas neuroendocrine markers synaptophysin (Syn) and INSM1 (Fig. 3D) were negative. Based on the gross, histomorphological features and immunohistochemical profile, a final diagnosis of SP was rendered.
Thoracic CT (A) scan displayed a single, well-circumscribed, solid, regular mass in the lower lobe of the right lung (white arrow). On intraoperative consultation, it was showed that the tumor cells were mainly in solid growth pattern (B, ×100). In these area, the distinct rosette structures similar to carcinoid were observed (C, ×100) (D, ×200).
The classical papillary growth pattern of SP with surface cuboidal cells and stromal round or polygonal cells were found in the permanent sections (A, ×100). The sclerotic (B, ×100) and rosette-shaped (C, ×200) growth pattern were also observed. The tumor cells in these rosette areas were relatively uniform without obvious atypia (D, ×400).
In the World Health Organization (WHO) classification (2021), SP is considered to be a benign tumor of pneumocyte origin. Radiographically, it commonly appears as a solitary, well-circumscribed, round to oval mass. Generally SP is restricted, non-encapsulated, easily exracted, solid nodules. This tumor is characterized by two distinct cell populations: surface cell populations and rounded stromal cells, often organized into four structural patterns: papillary, sclerotic, solid, and hemorrhagic. Both cell types typically show diffuse positivity for TTF-1, vimentin, and negativity for neuroendocrine markers. In addition, surface cells often are positive for Napsin A, cytokeratins AE1/AE3, CK7, CAM 5.2, and surfactant proteins A and B.3 This rare benign disease may present some diagnostic challenges because radiological features resemble those of malignancy, and preoperative and postoperative cytohistological studies may depict features similar to those of aggressive lung tumors (particularly adenocarcinomas or neuroendocrine tumors). It is generally believed that the most helpful diagnostic clues in the diagnosis of SP are generally well-circumscribed, easily shelled out, and easily identifiable multiple histological growth patterns.4 In addition, although carcinoid may also be a diagnostic pitfall, the histological rosette pattern is generally considered to be an important differentiator in addition to neuroendocrine markers.5 However, our particular case showed definite rosette structure mimicking carcinoid in intraoperative and postoperative sections, which may be a diagnostic pitfall, especially in intraoperative consultations. To the best of our knowledge, there is no English literature report on the occurrence of rosette structure in SP.
FundingThe authors have not received any funding.
Ethical considerationsWritten informed consent was obtained from the patient guardian for publication of the article.