Tuberculosis (TB) is associated with a high mortality in the intensive care unit (ICU), especially in subjects with Acute Respiratory Distress Syndrome (ARDS) requiring mechanical ventilation. Despite its global burden on morbidity and mortality, TB is an uncommon cause of ICU admission, however mortality is disproportionate to the advances in diagnosis and treatment made. Herein we report a systematic review of published studies.
MethodsOur Literature search was conducted to identify studies on outcomes of individuals with TB admitted to ICU. We report and review in-hospital mortality, predictors of poorer outcomes, usefulness of severity scoring systems and potential benefits of intravenous antibiotics. Searches from Pubmed, Embase, Cochrane and Medline were conducted from inception to March 2020. Only literature in English was included.
ResultsOut of 529 potentially relevant articles, 17 were included. Mortality across all studies ranged from 29-95% with an average of 52.9%. All severity scores underestimated average mortality. The most common indication for ICU admission was acute respiratory failure (36.3%). Negative predictors of outcome included hospital acquired infections, need of mechanical ventilation and vasopressors, delay in initiation of anti-TB treatment, more than one organ failure and a higher severity score. Low income, high incidence countries showed a 23.4% higher mortality rate compared to high income, low TB incidence countries.
ConclusionMortality in individuals with TB admitted to ICU is high. Earlier detection and treatment initiation is needed.
Tuberculosis (TB) was within the top ten causes of death and second cause of death from a single infectious agent worldwide in 2020.1–4 The aim of treatment is to reduce the incidence of resistance and achieve full bacterial clearance, thereby limiting the risk of transmission.5,6 Success of drug susceptible TB under trial conditions is up to 95% in non-critical subjects; this success is underpinned by adequate concentrations of these drugs in the blood.7
Although TB most commonly manifests sub-acutely or chronically, some individuals especially those with extensive disease may progress rapidly, requiring admission to intensive care unit (ICU). Up to 3% of all patients with TB require ICU admission, a high proportion considering the availability of curative treatment.8 The most common indication for ICU admission is respiratory failure and acute respiratory distress syndrome (ARDS).9–11 The mortality for TB patients admitted to ICU is extremely high, more than any other cause of respiratory failure including pneumonia.12 Reported mortality rates are variable across studies, and can range from 24% to 81% in individuals requiring mechanical ventilation.13 The mortality for ARDS secondary due to TB has not changed significantly over time, despite advances in new treatment regimens and ventlilatory strategies in ICU. The heterogeneity of disease presentation and the difficulty in diagnosis remain a challenge. Co-morbidities including HIV, immunosuppressive disorders and diabetes increase the risk of complications in patients with TB.9 Poor prognostic indicators include high Acute Physiology And Chronic Health Evaluation II (APACHE II) or Simplified Acute Physiology Score (SAPS) II scores, nosocomial infections, sepsis and delayed start of anti TB treatment.14 The full extent of the association of TB with Covid-19 and the risk of admission to ICU and the need for mechanical ventilation is currently not known.15–18
Delays in diagnosis and treatment of pulmonary TB are principal causes of death, especially in patients with acute respiratory failure.11,19,20 Early diagnosis and start of effective treatment is needed to prevent ICU admission and complications.9 It is imperative that the absorption of anti-TB treatment is maximised; a challenge in the critically ill individual. Deranged physiological functioning and poor gastric absorption can lead to sub-therapeutic drug levels.21 Intravenous antibiotics may overcome these obstacles. Despite the bioavailability of parenteral routes, the use of intravenous antimicrobials is seldom used in TB. If intravenous rifampicin, was more widely available, it may negate the need for more toxic regimens.
Severity scoring systems such as APACHE II have been proven to predict mortality in individuals admitted to ICU.22 This may not be the case for individuals with TB related ARDS and septic shock, as some studies have suggested they consistently underestimate mortality in these groups.23,24 The low prevalence of TB in ICU is a further challenge. In published studies, small sample sizes limit the potential generalisation of results.25 Further research and studies with larger patient groups are needed.
This review aims to identify factors affecting poor outcomes and mortality of individuals with pulmonary TB admitted to ICU. Further objectives include identifying factors leading to TB-related complications, the relevance of ICU severity scores and the role of using first line intravenous anti-TB drugs in critically ill subjects. We hypothesise that identifying predictors of poor outcomes in TB patients admitted to ICU can contribute to risk stratification and personalised treatment.
MethodsSearch strategyTo avoid any influence of the effects of pandemic on recent publications,26 Pubmed, EMBASE, Cochrane and Medline databases were searched from inception until March 2020. Keywords included: (“Outcome*” or “mortality” or “impact” or “recovery” or “effect*”) and (“Mycobacterium tuberculosis” or “tuberculosis” or “TB” or ‘MTB”) and (“intensive care unit*” or “intensive treatment unit*” or “critical care” or “CCU” or “ARDS” or “Acute respiratory distress syndrome” or “mechanical ventilation” or “respiratory failure”) and (“scor*” or “severity” or “APACHE” or “APACHEII” or “GCS” or “SOFA” or “SAPS” or “Charlson”), “Intravenous” or “antibiotic*” or “Rifampin” or “Isoniazid” or “ethambutol” or “Pyrazinamide”.
Study selectionPublished studies were included if they reported on outcomes of cohorts of patients with pulmonary TB admitted to ICU Studies involving, individuals < 18 years and those involving <10 patients were excluded. Conference abstracts, posters, patient case studies and articles with no reported outcomes were excluded.
In the first stage, we screened the titles and abstracts of all citations for potentially relevant papers. In the second stage, we examined in detail the full texts of the retrieved papers.
Data extractionInformation on study design, setting, population characteristics including comorbidities, reason for ICU admission as well as ICU outcomes were obtained (see Table 1). Factors affecting outcomes were also recorded in a separate Table 2, and including information on mechanical ventilation, length of hospital and ICU stay, ICU related complications were obtained. Tuberculosis related outcomes such as time to initiation of anti-TB treatment, drug susceptibility pattern, concomitant treatments, were recorded (Table 2). All ICU related severity scores were recorded. (Table 3).
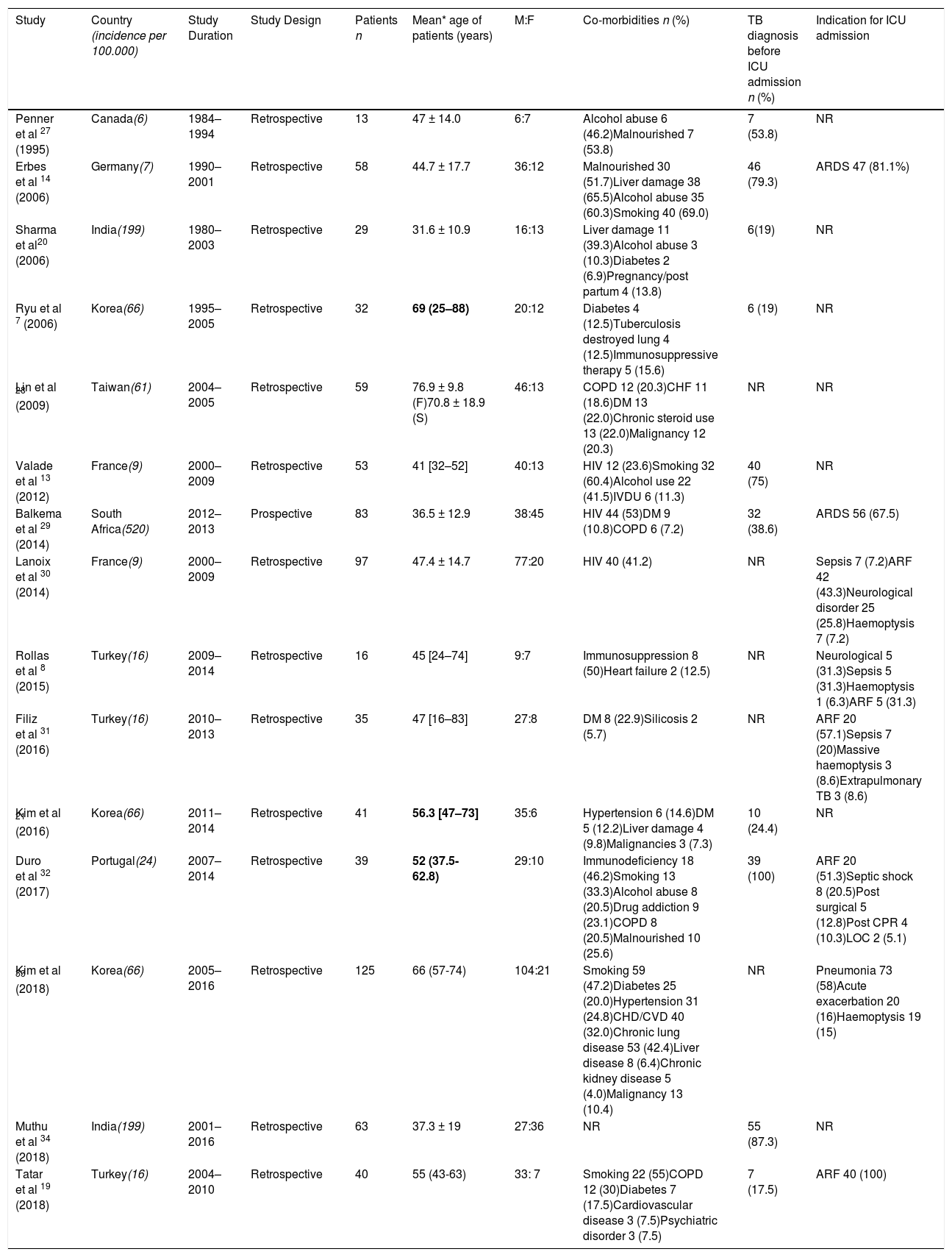
Studies of patients with tuberculosis in the intensive care setting.
| Study | Country (incidence per 100.000) | Study Duration | Study Design | Patients n | Mean* age of patients (years) | M:F | Co-morbidities n (%) | TB diagnosis before ICU admission n (%) | Indication for ICU admission |
|---|---|---|---|---|---|---|---|---|---|
| Penner et al 27 (1995) | Canada(6) | 1984–1994 | Retrospective | 13 | 47 ± 14.0 | 6:7 | Alcohol abuse 6 (46.2)Malnourished 7 (53.8) | 7 (53.8) | NR |
| Erbes et al 14 (2006) | Germany(7) | 1990–2001 | Retrospective | 58 | 44.7 ± 17.7 | 36:12 | Malnourished 30 (51.7)Liver damage 38 (65.5)Alcohol abuse 35 (60.3)Smoking 40 (69.0) | 46 (79.3) | ARDS 47 (81.1%) |
| Sharma et al20 (2006) | India(199) | 1980–2003 | Retrospective | 29 | 31.6 ± 10.9 | 16:13 | Liver damage 11 (39.3)Alcohol abuse 3 (10.3)Diabetes 2 (6.9)Pregnancy/post partum 4 (13.8) | 6(19) | NR |
| Ryu et al 7 (2006) | Korea(66) | 1995–2005 | Retrospective | 32 | 69 (25–88) | 20:12 | Diabetes 4 (12.5)Tuberculosis destroyed lung 4 (12.5)Immunosuppressive therapy 5 (15.6) | 6 (19) | NR |
| Lin et al 28 (2009) | Taiwan(61) | 2004–2005 | Retrospective | 59 | 76.9 ± 9.8 (F)70.8 ± 18.9 (S) | 46:13 | COPD 12 (20.3)CHF 11 (18.6)DM 13 (22.0)Chronic steroid use 13 (22.0)Malignancy 12 (20.3) | NR | NR |
| Valade et al 13 (2012) | France(9) | 2000–2009 | Retrospective | 53 | 41 [32–52] | 40:13 | HIV 12 (23.6)Smoking 32 (60.4)Alcohol use 22 (41.5)IVDU 6 (11.3) | 40 (75) | NR |
| Balkema et al 29 (2014) | South Africa(520) | 2012–2013 | Prospective | 83 | 36.5 ± 12.9 | 38:45 | HIV 44 (53)DM 9 (10.8)COPD 6 (7.2) | 32 (38.6) | ARDS 56 (67.5) |
| Lanoix et al 30 (2014) | France(9) | 2000–2009 | Retrospective | 97 | 47.4 ± 14.7 | 77:20 | HIV 40 (41.2) | NR | Sepsis 7 (7.2)ARF 42 (43.3)Neurological disorder 25 (25.8)Haemoptysis 7 (7.2) |
| Rollas et al 8 (2015) | Turkey(16) | 2009–2014 | Retrospective | 16 | 45 [24–74] | 9:7 | Immunosuppression 8 (50)Heart failure 2 (12.5) | NR | Neurological 5 (31.3)Sepsis 5 (31.3)Haemoptysis 1 (6.3)ARF 5 (31.3) |
| Filiz et al 31 (2016) | Turkey(16) | 2010–2013 | Retrospective | 35 | 47 [16–83] | 27:8 | DM 8 (22.9)Silicosis 2 (5.7) | NR | ARF 20 (57.1)Sepsis 7 (20)Massive haemoptysis 3 (8.6)Extrapulmonary TB 3 (8.6) |
| Kim et al 21 (2016) | Korea(66) | 2011–2014 | Retrospective | 41 | 56.3 [47–73] | 35:6 | Hypertension 6 (14.6)DM 5 (12.2)Liver damage 4 (9.8)Malignancies 3 (7.3) | 10 (24.4) | NR |
| Duro et al 32 (2017) | Portugal(24) | 2007–2014 | Retrospective | 39 | 52 (37.5- 62.8) | 29:10 | Immunodeficiency 18 (46.2)Smoking 13 (33.3)Alcohol abuse 8 (20.5)Drug addiction 9 (23.1)COPD 8 (20.5)Malnourished 10 (25.6) | 39 (100) | ARF 20 (51.3)Septic shock 8 (20.5)Post surgical 5 (12.8)Post CPR 4 (10.3)LOC 2 (5.1) |
| Kim et al 33 (2018) | Korea(66) | 2005–2016 | Retrospective | 125 | 66 (57-74) | 104:21 | Smoking 59 (47.2)Diabetes 25 (20.0)Hypertension 31 (24.8)CHD/CVD 40 (32.0)Chronic lung disease 53 (42.4)Liver disease 8 (6.4)Chronic kidney disease 5 (4.0)Malignancy 13 (10.4) | NR | Pneumonia 73 (58)Acute exacerbation 20 (16)Haemoptysis 19 (15) |
| Muthu et al 34 (2018) | India(199) | 2001–2016 | Retrospective | 63 | 37.3 ± 19 | 27:36 | NR | 55 (87.3) | NR |
| Tatar et al 19 (2018) | Turkey(16) | 2004–2010 | Retrospective | 40 | 55 (43-63) | 33: 7 | Smoking 22 (55)COPD 12 (30)Diabetes 7 (17.5)Cardiovascular disease 3 (7.5)Psychiatric disorder 3 (7.5) | 7 (17.5) | ARF 40 (100) |
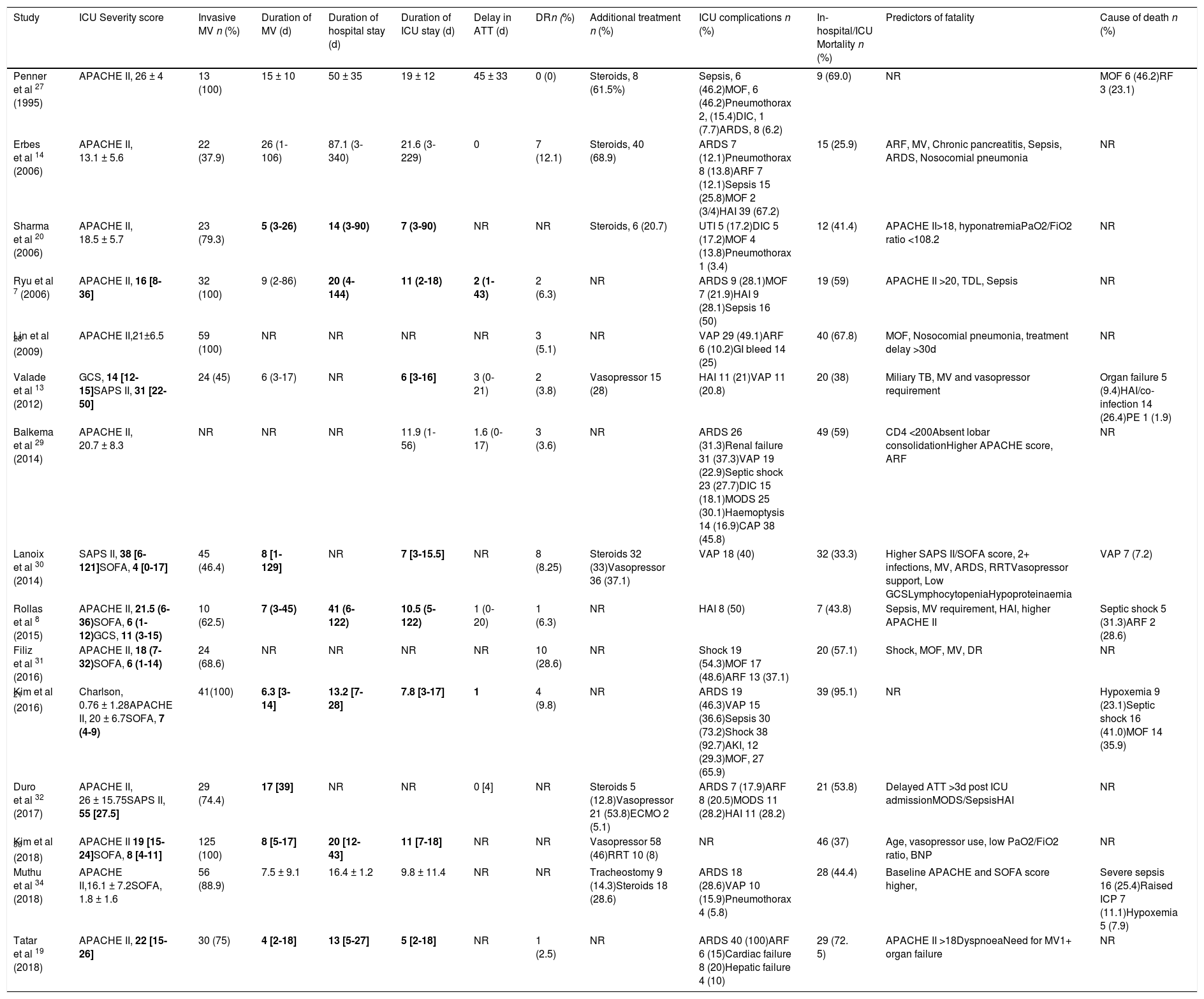
Summary of studies showing patient variables and outcomes in ICU.
| Study | ICU Severity score | Invasive MV n (%) | Duration of MV (d) | Duration of hospital stay (d) | Duration of ICU stay (d) | Delay in ATT (d) | DRn (%) | Additional treatment n (%) | ICU complications n (%) | In-hospital/ICU Mortality n (%) | Predictors of fatality | Cause of death n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penner et al 27 (1995) | APACHE II, 26 ± 4 | 13 (100) | 15 ± 10 | 50 ± 35 | 19 ± 12 | 45 ± 33 | 0 (0) | Steroids, 8 (61.5%) | Sepsis, 6 (46.2)MOF, 6 (46.2)Pneumothorax 2, (15.4)DIC, 1 (7.7)ARDS, 8 (6.2) | 9 (69.0) | NR | MOF 6 (46.2)RF 3 (23.1) |
| Erbes et al 14 (2006) | APACHE II, 13.1 ± 5.6 | 22 (37.9) | 26 (1-106) | 87.1 (3-340) | 21.6 (3-229) | 0 | 7 (12.1) | Steroids, 40 (68.9) | ARDS 7 (12.1)Pneumothorax 8 (13.8)ARF 7 (12.1)Sepsis 15 (25.8)MOF 2 (3/4)HAI 39 (67.2) | 15 (25.9) | ARF, MV, Chronic pancreatitis, Sepsis, ARDS, Nosocomial pneumonia | NR |
| Sharma et al 20 (2006) | APACHE II, 18.5 ± 5.7 | 23 (79.3) | 5 (3-26) | 14 (3-90) | 7 (3-90) | NR | NR | Steroids, 6 (20.7) | UTI 5 (17.2)DIC 5 (17.2)MOF 4 (13.8)Pneumothorax 1 (3.4) | 12 (41.4) | APACHE II>18, hyponatremiaPaO2/FiO2 ratio <108.2 | NR |
| Ryu et al 7 (2006) | APACHE II, 16 [8-36] | 32 (100) | 9 (2-86) | 20 (4-144) | 11 (2-18) | 2 (1-43) | 2 (6.3) | NR | ARDS 9 (28.1)MOF 7 (21.9)HAI 9 (28.1)Sepsis 16 (50) | 19 (59) | APACHE II >20, TDL, Sepsis | NR |
| Lin et al 28 (2009) | APACHE II,21±6.5 | 59 (100) | NR | NR | NR | NR | 3 (5.1) | NR | VAP 29 (49.1)ARF 6 (10.2)GI bleed 14 (25) | 40 (67.8) | MOF, Nosocomial pneumonia, treatment delay >30d | NR |
| Valade et al 13 (2012) | GCS, 14 [12-15]SAPS II, 31 [22-50] | 24 (45) | 6 (3-17) | NR | 6 [3-16] | 3 (0-21) | 2 (3.8) | Vasopressor 15 (28) | HAI 11 (21)VAP 11 (20.8) | 20 (38) | Miliary TB, MV and vasopressor requirement | Organ failure 5 (9.4)HAI/co-infection 14 (26.4)PE 1 (1.9) |
| Balkema et al 29 (2014) | APACHE II, 20.7 ± 8.3 | NR | NR | NR | 11.9 (1-56) | 1.6 (0-17) | 3 (3.6) | NR | ARDS 26 (31.3)Renal failure 31 (37.3)VAP 19 (22.9)Septic shock 23 (27.7)DIC 15 (18.1)MODS 25 (30.1)Haemoptysis 14 (16.9)CAP 38 (45.8) | 49 (59) | CD4 <200Absent lobar consolidationHigher APACHE score, ARF | NR |
| Lanoix et al 30 (2014) | SAPS II, 38 [6-121]SOFA, 4 [0-17] | 45 (46.4) | 8 [1-129] | NR | 7 [3-15.5] | NR | 8 (8.25) | Steroids 32 (33)Vasopressor 36 (37.1) | VAP 18 (40) | 32 (33.3) | Higher SAPS II/SOFA score, 2+ infections, MV, ARDS, RRTVasopressor support, Low GCSLymphocytopeniaHypoproteinaemia | VAP 7 (7.2) |
| Rollas et al 8 (2015) | APACHE II, 21.5 (6-36)SOFA, 6 (1-12)GCS, 11 (3-15) | 10 (62.5) | 7 (3-45) | 41 (6-122) | 10.5 (5-122) | 1 (0-20) | 1 (6.3) | NR | HAI 8 (50) | 7 (43.8) | Sepsis, MV requirement, HAI, higher APACHE II | Septic shock 5 (31.3)ARF 2 (28.6) |
| Filiz et al 31 (2016) | APACHE II, 18 (7-32)SOFA, 6 (1-14) | 24 (68.6) | NR | NR | NR | NR | 10 (28.6) | NR | Shock 19 (54.3)MOF 17 (48.6)ARF 13 (37.1) | 20 (57.1) | Shock, MOF, MV, DR | NR |
| Kim et al 21 (2016) | Charlson, 0.76 ± 1.28APACHE II, 20 ± 6.7SOFA, 7 (4-9) | 41(100) | 6.3 [3-14] | 13.2 [7-28] | 7.8 [3-17] | 1 | 4 (9.8) | NR | ARDS 19 (46.3)VAP 15 (36.6)Sepsis 30 (73.2)Shock 38 (92.7)AKI, 12 (29.3)MOF, 27 (65.9) | 39 (95.1) | NR | Hypoxemia 9 (23.1)Septic shock 16 (41.0)MOF 14 (35.9) |
| Duro et al 32 (2017) | APACHE II, 26 ± 15.75SAPS II, 55 [27.5] | 29 (74.4) | 17 [39] | NR | NR | 0 [4] | NR | Steroids 5 (12.8)Vasopressor 21 (53.8)ECMO 2 (5.1) | ARDS 7 (17.9)ARF 8 (20.5)MODS 11 (28.2)HAI 11 (28.2) | 21 (53.8) | Delayed ATT >3d post ICU admissionMODS/SepsisHAI | NR |
| Kim et al 33 (2018) | APACHE II 19 [15-24]SOFA, 8 [4-11] | 125 (100) | 8 [5-17] | 20 [12-43] | 11 [7-18] | NR | NR | Vasopressor 58 (46)RRT 10 (8) | NR | 46 (37) | Age, vasopressor use, low PaO2/FiO2 ratio, BNP | NR |
| Muthu et al 34 (2018) | APACHE II,16.1 ± 7.2SOFA, 1.8 ± 1.6 | 56 (88.9) | 7.5 ± 9.1 | 16.4 ± 1.2 | 9.8 ± 11.4 | NR | NR | Tracheostomy 9 (14.3)Steroids 18 (28.6) | ARDS 18 (28.6)VAP 10 (15.9)Pneumothorax 4 (5.8) | 28 (44.4) | Baseline APACHE and SOFA score higher, | Severe sepsis 16 (25.4)Raised ICP 7 (11.1)Hypoxemia 5 (7.9) |
| Tatar et al 19 (2018) | APACHE II, 22 [15-26] | 30 (75) | 4 [2-18] | 13 [5-27] | 5 [2-18] | NR | 1 (2.5) | NR | ARDS 40 (100)ARF 6 (15)Cardiac failure 8 (20)Hepatic failure 4 (10) | 29 (72. 5) | APACHE II >18DyspnoeaNeed for MV1+ organ failure | NR |
n= number of patients (d)=days
Incidence is reported as estimated rate of tuberculosis per 100,000 from gov.org last updated 2019 (high incidence is > 40/100,000)
All averages are mean ± SD unless stated otherwise; Median is signified in bold with (range) or [IQR]
APACHE II is worst score in 24 h of admission
Mortality is reported as ‘in-hospital mortality’ unless stated otherwise
F: fatalities
S: survivors
NR: data not reported
MV: mechanical ventilation
DR%: percentage of patients with drug resistant strains
ATT= anti-tuberculosis treatment
ARDS= acute respiratory distress syndrome
VAP=ventilator assisted pneumonia
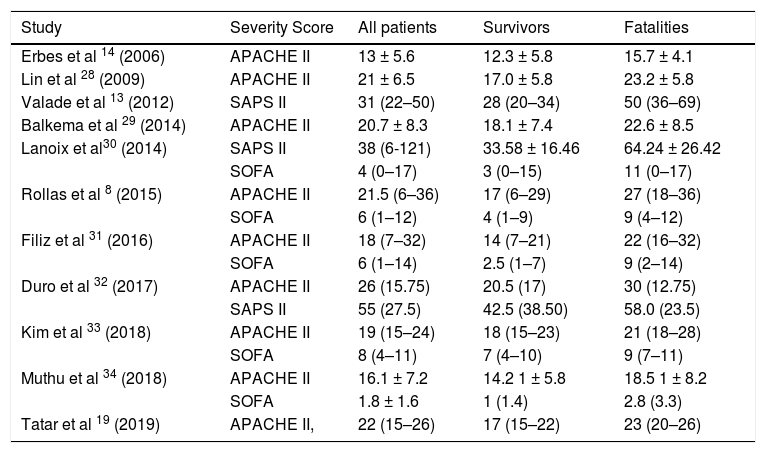
Severity scoring for survivors vs fatalities.
| Study | Severity Score | All patients | Survivors | Fatalities |
|---|---|---|---|---|
| Erbes et al 14 (2006) | APACHE II | 13 ± 5.6 | 12.3 ± 5.8 | 15.7 ± 4.1 |
| Lin et al 28 (2009) | APACHE II | 21 ± 6.5 | 17.0 ± 5.8 | 23.2 ± 5.8 |
| Valade et al 13 (2012) | SAPS II | 31 (22–50) | 28 (20–34) | 50 (36–69) |
| Balkema et al 29 (2014) | APACHE II | 20.7 ± 8.3 | 18.1 ± 7.4 | 22.6 ± 8.5 |
| Lanoix et al30 (2014) | SAPS II | 38 (6-121) | 33.58 ± 16.46 | 64.24 ± 26.42 |
| SOFA | 4 (0–17) | 3 (0–15) | 11 (0–17) | |
| Rollas et al 8 (2015) | APACHE II | 21.5 (6–36) | 17 (6–29) | 27 (18–36) |
| SOFA | 6 (1–12) | 4 (1–9) | 9 (4–12) | |
| Filiz et al 31 (2016) | APACHE II | 18 (7–32) | 14 (7–21) | 22 (16–32) |
| SOFA | 6 (1–14) | 2.5 (1–7) | 9 (2–14) | |
| Duro et al 32 (2017) | APACHE II | 26 (15.75) | 20.5 (17) | 30 (12.75) |
| SAPS II | 55 (27.5) | 42.5 (38.50) | 58.0 (23.5) | |
| Kim et al 33 (2018) | APACHE II | 19 (15–24) | 18 (15–23) | 21 (18–28) |
| SOFA | 8 (4–11) | 7 (4–10) | 9 (7–11) | |
| Muthu et al 34 (2018) | APACHE II | 16.1 ± 7.2 | 14.2 1 ± 5.8 | 18.5 1 ± 8.2 |
| SOFA | 1.8 ± 1.6 | 1 (1.4) | 2.8 (3.3) | |
| Tatar et al 19 (2019) | APACHE II, | 22 (15–26) | 17 (15–22) | 23 (20–26) |
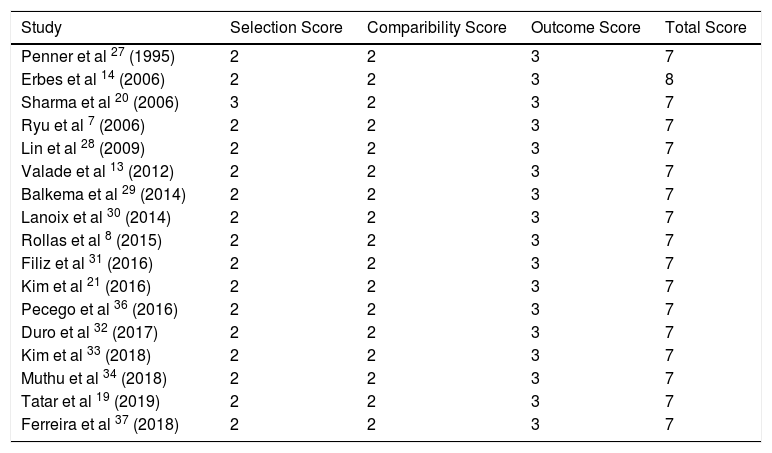
The Newcastle-Ottawa assessment scale (NOS) for cohort studies was used to assess study quality and risk of bias.35 The Newcastle-Ottawa assessment scale evaluates three parameters; selection, comparability and outcome, awarding a certain number of points. The maximum number a study can receive is 9 points, indicating low risk of bias. Less than 5 points indicate a high risk of bias. The outcome used for the checklist was mortality.
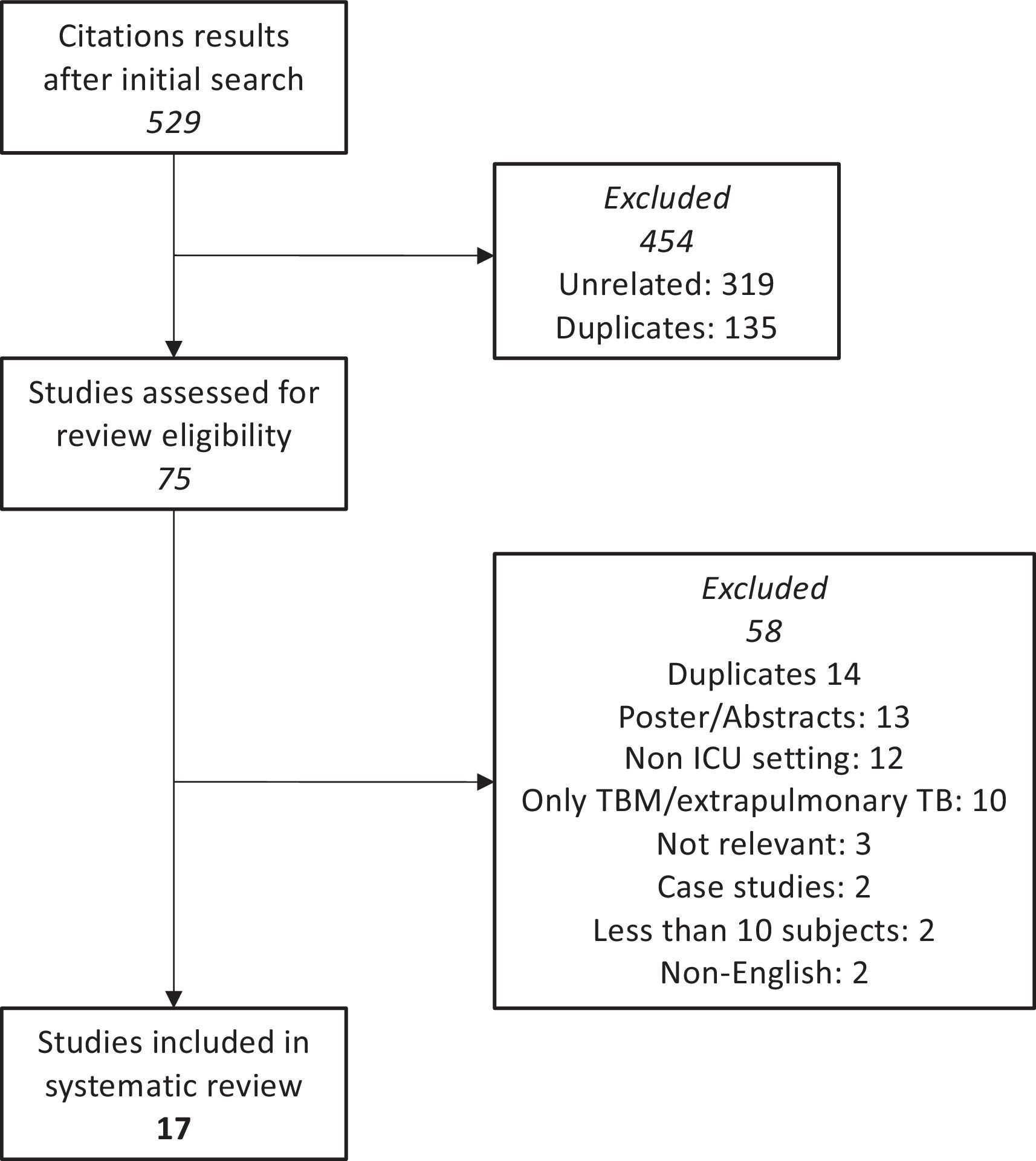
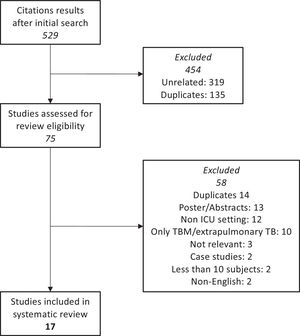
Fig. 1. PRISMA flow diagram of selected studies.
ResultsCharacteristics of the studiesSeventeen out of 529 studies fulfilled the inclusion criteria and were included in the review. The studies ranged from 1995 to 2018. The studies included were from high (South Africa, South Korea, India) and low/intermediate TB incidence countries (Canada, Germany, Taiwan, France, Turkey, Portugal). All studies were retrospective except Balkema et al.29 which was prospective. A total of 947 cases with active pulmonary TB who required ICU admission were included across all studies, of which 652 were male.
Quality of studiesQuality of studies was generally high when assessed using NOS checklist. Selection bias across studies was greatest risk due to clinician selected cohort groups, with small sample sizes. All had follow up resulting in outcomes with all subjects accounted for, and outcomes were clearly defined in all studies. No study had an overall outcome <7 points indicating low risk of bias (Table 4).
Bias according to the Newcastle-Ottawa assessment scale.35
| Study | Selection Score | Comparibility Score | Outcome Score | Total Score |
|---|---|---|---|---|
| Penner et al 27 (1995) | 2 | 2 | 3 | 7 |
| Erbes et al 14 (2006) | 2 | 2 | 3 | 8 |
| Sharma et al 20 (2006) | 3 | 2 | 3 | 7 |
| Ryu et al 7 (2006) | 2 | 2 | 3 | 7 |
| Lin et al 28 (2009) | 2 | 2 | 3 | 7 |
| Valade et al 13 (2012) | 2 | 2 | 3 | 7 |
| Balkema et al 29 (2014) | 2 | 2 | 3 | 7 |
| Lanoix et al 30 (2014) | 2 | 2 | 3 | 7 |
| Rollas et al 8 (2015) | 2 | 2 | 3 | 7 |
| Filiz et al 31 (2016) | 2 | 2 | 3 | 7 |
| Kim et al 21 (2016) | 2 | 2 | 3 | 7 |
| Pecego et al 36 (2016) | 2 | 2 | 3 | 7 |
| Duro et al 32 (2017) | 2 | 2 | 3 | 7 |
| Kim et al 33 (2018) | 2 | 2 | 3 | 7 |
| Muthu et al 34 (2018) | 2 | 2 | 3 | 7 |
| Tatar et al 19 (2019) | 2 | 2 | 3 | 7 |
| Ferreira et al 37 (2018) | 2 | 2 | 3 | 7 |
To aid an inclusive qualitative analysis, the averages of medians and means were calculated, with each study weighted equally, regardless, of the number of cases. The mean or median age of cases ranged between 31.6–76.9 years with 12/17 studies having a mean/median age > 41 years. Common comorbidities included HIV co-infection (27.1%), alcohol abuse (12.5%), diabetes (7.7%) and malnourishment (5.0%). 21% of cases were smokers. Thirty-eight% of cases had a diagnosis of TB prior to ICU admission. The most common indication for ICU admission was respiratory failure and ARDS (36.3%) followed by pneumonia (9.3%), sepsis (4.3%) and massive haemoptysis (3.8%).
Acute Physiology and Chronic Health Evaluation II was the most commonly used scoring system, reported in 13 studies, however SAPS II, quick Sequential Organ Failure Assessment (qSOFA) which identifies high-risk patients for in-hospital mortality with suspected infection outside the ICU and the Glasgow Coma Scale (GCS) were also used across the studies. The average of the mean APACHE II score was 20.2 and median 19.1, across 8 and 6 studies, respectively. The average of the median SAPS II score was 42.8 across 4 studies and median SOFA was 5.8 across 6 studies. The mean and median values for severity scores were consistently higher in fatalities than survivors in all studies except for Pecego et al. with survivors having a higher SAPS II score (Table 3).36 The average of the mean APACHE II score was 22 and 16.4 for fatalities and survivors, respectively across 5 studies. The average of the median of these scores were 23.3 and 16.5 for fatalities and survivors, respectively, across 5 studies.
Individuals requiring mechanical ventilation ranged from 37.5% to 100%. Across all studies 67.2% of cases required mechanical ventilation and the duration in days was 14.5 and 13.25 days for the median and mean values, respectively. There was a large variation for example Erbes et al. provide a mean of 26 and a range of 1-106,14 similarly Lanoix et al.30 provide a median of 8 with an interquartile range (IQR) of 1-129.
Duration of hospital stay was reported in 9 studies. The average of the median was 20.2 days across 6 studies and for the mean 51.2 days across 3 studies. The duration of ICU stay was reported in 14 studies, the average of the median was 7.8 days across 9 studies and mean was 15.6 across 4 studies. Similarly, for the duration of stay for both hospital and ICU, there was a large spread of data throughout some studies, reflected by the large interquartile ranges in Table 2.
Delay in initiation of anti TB treatment (ATT) within hospital was only reported in 8 studies, the lowest being 0 days and the largest mean value was 45 days in Penner et al.27 The prevalence of drug resistance pattern was reported in 11 studies and ranged between 0% to 28.6%, 4.9% of cases having drug resistant strains when combining all studies. Steroids were given to 11.5% of cases and vasopressor support was given to 15.0% of cases. Other treatment management was given to a smaller number of individuals including extracorporeal membrane oxygenation, tracheostomy and renal replacement therapy.
The most common reported complication was ARDS affecting 19.5% of all cases, followed by ventilator associated pneumonia (10.8%), multiple organ failure (10.5%), sepsis (9.5%) and hospital acquired infections (8.2%). Other reported complications included shock, disseminated intravascular coagulation, acute kidney infection, single organ failure and pneumothorax. In-hospital mortality ranged from 29% to 95.1% giving a mortality rate of 52.9% across all studies. In two studies a lower ratio of arterial oxygen tension to fractional inspired oxygen (PaO2/FiO2) indicated a poorer prognosis.38,33 Causes of death were reported in 6 studies with septic shock and organ failure (including respiratory failure) with, respective values of 4.7% and 3.8% of total cases as the most common causes. Other causes of death included hospital acquired infection, raised intracranial pressure, pulmonary embolism and hypoxaemia.
No studies using first line intravenous anti TB medications in ICU were found.
DiscussionAcute respiratory failure, although a rare complication of TB, carries a high fatality rate. There is little research focus on the outcomes and factors affecting mortality in these patient groups, thereby hindering the ability of clinicians to change clinical practice and improve prognosis.
Mortality and ARDSIn this systematic review, average in-hospital mortality across 17 studies was 52.9%. This value is especially high considering availability and efficacy of ATT worldwide and the advancements in intensive care medicine. Attributable factors include delay in diagnosis and ATT initiation, altered drug absorption in critically ill patients, comorbidities and TB related complications. The most common complication and indication for ICU admission across the studies was found to be ARDS/acute hypoxaemic respiratory failure. Tuberculosis related acute respiratory failure carries a mortality rate of up to 60%, pneumonia carries a 25% mortality.38–40
Multiple organ failure and sepsis were present in 10.5% and 9.5% of cases, respectively and were included as predictors of mortality in 9 studies. Three studies documented individuals with disseminated intravascular coagulation. This can be caused by miliary TB and is a negative predictor for survival, with individuals more likely to develop ARDS than those with isolated pulmonary TB; it carries a high mortality in the ICU setting, mostly attributed to septicaemia and subsequent multiple organ failure.10 HIV/AIDS, alcohol abuse, diabetes, smoking status and chronic pancreatitis identified as independent risk factors for mortality.14
HIV and TBTuberculosis is the main cause of death in people living with HIV.41 People living with HIV are 30 times more likely to develop active TB, with more severe and atypical pulmonary forms as the most common presentation.37 Two studies reported an earlier age of hospitalisation and higher rate of respiratory failure.36,37 Threshold for clinical suspicion should be lower in these individuals, given their diminished symptom presentation.12,42
ICU complicationsSeveral ICU complications were reported. Critically unwell individuals are prone to drug interactions and adverse effects due to complex pharmacology, polypharmacy, disease severity and organ failure.43 Hepatotoxicity is a particular risk with isoniazid, rifampicin and pyrazinamide. Patients with underlying hepatic sequelae including prior hepatitis, alcoholic liver disease are more vulnerable. Acute kidney injury and glomerular hyperfiltration can affect anti-TB drug elimination with pyrazinamide and ethambutol renally excreted.44 Decompensated or end stage renal failure in ICU negatively influences patient outcome especially in those requiring dialysis, 45 individuals across these studies had renal failure.14 Patients in multiple organ failure are less tolerant to the toxic side effects of anti-TB drugs, creating clinical dilemmas as therapy interruption can increase risk of drug resistance and death.
Hospital acquired infections (HAIs) were found to be a negative predictor of survival and were present in 8.2% of cases. Tuberculosis suppresses monocyte activity, causing immunosuppression and increasing infection risk.34 Lin et al reported nosocomial pneumonia incidence was four times higher in non-surviving individuals with pulmonary TB.28 Ventilator associated pneumonia was found in numerous individuals who had been ventilated,14 and was independently associated with hospital mortality.14 Other infections include urinary tract and central venous catheter associated bloodstream infections which are associated with length hospital stay. Hospital acquired infections can prolong length of stay, contributing to an already elevated mortality rate.45
Diagnostic delaySmear microscopy and culture have turnaround times of few days and several weeks, respectively. GeneXpert NAAT TB-PCR test and urinary LAM (Fujifilm) may allow for results within hours.9 Despite the growing availability of fast and reliable point of care tests, thinking of TB remains a challenge. Misinterpretation of clinical and radiological presentation, and lack of resources contribute to unreliable diagnosis and delays in treatment initiation. It can be challenging to radiologically distinguish TB from severe bacterial pneumonia as a cause of ARDS, many individuals are treated incorrectly before TB is considered in the differential. Empirical fluroquinolone could be beneficial covering both conditions, Tseng et al reported oral fluoroquinolone usage as independently associated with better survival in those with TB mimicking severe pneumonia in ICU.46
Survival of individuals with TB can be significantly improved if therapy is started within 14 days of hospitalisation.46 Erbes et al found a significant increase in mortality in individuals not receiving optimal treatment including isoniazid and rifampicin.14 In addition, Duro et al found that starting ATT within 3 days of ICU admission improved survival.32 Two studies with the longest delay in treatment initiation were from lower incidence countries.13,27 Delays are common in areas with fewer TB cases, probably as a result of lack of experience.47 Almost half of the studies did not report on treatment delay. The variation in delay ranged from 0-45 days globally, and may contribute to poorer prognosis. This review found only 38.3% of individuals diagnosed prior to admission.
Drug resistanceRifampicin resistance is increasing and a major threat, with half a million people currently estimated to be infected with rifampicin resistant strains carrying a higher mortality.2,24 The number of individuals with drug resistant TB was 46 (4.9%). Drug resistance may have been under reported in these studies and this might explain why resistance was not found to be a predictor of mortality.
Intravenous anti-TB treatmentTuberculosis treatment in ICU is complicated by organ dysfunction, drug toxicity and sub-therapeutic levels. First line drugs such as rifampicin and isoniazid are generally well absorbed when administered orally at the correct dose. In critically unwell individuals, absorption and pharmacokinetic drug properties are altered. The pharmacokinetic profile of anti-TB drugs has shown that there is a dose dependent relationship between concentration and clinical outcomes.48 Critical illness alters gut motility, impairs mucosal barrier integrity, distorts commensal flora, delays gastric emptying leading to reduced absorption.10,25,49 Hypoalbuminemia was found to be a predictor of mortality in this review with 47 individuals suffering from malnutrition pre-admission.37 Hypoalbuminemia may lead to oedema, increasing the volume of distribution of drugs, as well as impair drug absorption all leading to lower drug concentrations in serum.44,50 Parenteral administration or higher doses of drugs may be required to reach therapeutic effect.
Although no studies regarding intravenous antibiotics were found, a study by Hill suggested a role for their use.25 They compared patient groups over 2 weeks, administering standard oral versus a 33% higher dose of intravenous rifampicin, finding a three times higher ‘geometric mean area under the time concentration curve’ up to 6 h, in the intravenous group. Mortality was substantially lower in individuals given intravenous rifampicin with no reported increase in toxicity. They also found an increased survival compared to the standard oral dose, including more rapid resolution of coma and reduced mortality at 2 months and 8 months.25 Koegelenberg et al investigated the pharmacokinetics of enteral anti-TB drugs in intensive care individuals, finding that a fixed dose of rifampicin administered via nasogastric tube resulted in sub-therapeutic plasma concentrations in the majority of individuals.48
Although intravenous rifampicin is available, it is not widely accessible in low income countries.51 Other first line drugs are not always accessible or available,48 with no intravenous ATT formulation included current WHO Model List of Essential Medicines (2019).52 This leads to use of second line drugs such as fluroquinolones and aminoglycosides in the ICU setting.53
Mechanical ventilation and steroidsSeveral studies identified mechanical ventilation as a risk factor for mortality.8,30 The four highest mortality rates reported were from Kim et al. 2016 (95.1%),21 Ferreira et al. (78.3%),37 Tatar et al. (72.5%),19 and Penner et al. (69.0%),27 having the highest proportion of mechanically ventilated individuals (75-100%). Studies with the lowest proportion of mechanically ventilated individuals had the lowest reported mortality, such as Erbes et al.14 with 37% ventilated and 25.9% mortality.13,14,30 Those with more severe, disseminated forms of disease were more likely to require mechanical ventilation and develop ARDS, reflecting a referral bias, most unwell more likely to die.12 Duration of mechanical ventilation has been associated with worse prognosis, possibly due to more HAIs, and pneumothorax.14
Adjuvant corticosteroid use is indicated for meningeal and pericardial disease, and pulmonary TB related ARDS.12,32 Some studies have shown that systemic glucocorticoids are associated with improved prognosis, however this was non-specific for the critically unwell population.54 The benefit of steroid use in TB individuals in ICU specifically remains unclear. We found that steroid use did not alter prognosis. Vasopressor support was found to be a predictor of fatality.
Severity scoring systems in ICUScoring systems for critically ill individuals are commonly used for estimating general ICU mortality, guiding clinical decision making and influencing distribution of hospital resources.55 Individuals with a higher mortality risk may benefit from earlier, targeted and potentially more aggressive treatment, given the small intervention window and a higher risk of death; this may outweigh risk of iatrogenic harm.56
Many studies have shown APACHE II and SAPS consistently underestimate mortality among individuals with pulmonary TB, especially those with ARDS and the mechanically ventilated.55,31 This highlights a shortfall in accurate risk stratification in these individuals, with a need for better tailored, ARDS specific scoring systems. APACHE does not include mechanical ventilation as an adverse outcome predictor a factor in its inaccuracy.22 In the literature it has been reported than an APACHE score >18 is associated with a higher mortality giving a predicted mortality of >29%.44 The average of mean APACHE II produced about 36% predicted mortality and using median a value about 32%. The median SAPS II and SOFA scores gave an estimated about 25% and <10%, respectively. Most of these results drastically underestimate the calculated mortality of 52.9%. The data set in Table 3 showed that the fatalities vs survivors had a higher score throughout (except for Pecago et al. 36).
Villar et al. designed an outcome score calculating 24hr post ARDS diagnosis, age, PaO2/FiO2 and plateau pressure.56,57 Similarly Kim et al. developed a mortality prediction model for individuals with TB-destroyed lung on mechanical ventilation.33 This model included age, vasopressor use, PaO2/FiO2 ratio and Brain Natriuretic Peptide (all predictors of ICU mortality in these individuals) finding this score more accurate at mortality prediction than APACHE II and SOFA.33 Lung injury severity 24 h after ARDS onset is a key determinant of outcome, reflecting the necessity for a reliable mortality prediction.56 Two studies found a low PaO2/FiO2 ratio to be a predictor of fatality.38,33 Although promising results have been obtained, further studies with perhaps additional variables are needed for external validation.58
High vs low burden areasNine out of the 17 studies were from high burden areas. Percentage of individuals diagnosed before admission was higher in low prevalence, resource rich areas, ranging from 53.8% to 75% over 4 studies (one not reported).13,14,30,27 In comparison to 24.4% to 38.6% (two not reported) over 4 studies,21,29,33,28 showing that more individuals are living with undiagnosed tuberculosis in poorer areas. This difference may be due to better diagnostic tools available in wealthier regions. The mortality in the low prevalence areas was 41.5% compared to the high prevalence at 64.9% with the highest mortality being the Kim et al 2016 study at 95.1%.21 The association between TB and low-income areas is known, with poverty being a cause and consequence of infection. Many risk factors for disease reactivation and predictors of mortality in ICU are associated with a lower socio-economic background, including HIV infection, malnutrition, alcohol use disorder and smoking.
More individuals were mechanically ventilated in high prevalence areas with higher mortality. Mechanical ventilation remains a predictor of mortality even in low burden areas. In these areas renal failure, sepsis, ARDS and APACHE II scoring are non-specific risk factors to TB.30 There was no difference in the APACHE II score, in contrast to the differing mortality rates between the grouped studies. This may reflect the inefficiency of severity scoring systems to accurately estimate mortality in critically unwell TB individuals.
LimitationsThis review only included individuals admitted to ICU which may reflect referral bias, as some lower income countries may not have had access to ICU beds. There was study heterogeneity in data reported, making meta -analysis challenging. No publication bias was assessed due to small sample sizes. No long term outcomes were reported.
ConclusionThe results across this review and previous literature are varied, reflecting the heterogeneity of patient presentation and aetiology of illness. The studies had relatively small sample sizes sand all save one were retrospective. There was disproportionate and variable mortality across studies only one-third of individuals were accurately diagnosed initially and 5% completed treatment successfully, highlighting the overwhelmingly poor outcomes for these individuals. A large number of individuals are undiagnosed until acutely unwell, leaving a small window for prompt diagnosis and treatment. Therapeutic intervention might be improved by administration of intravenous ATT, and may reduce complications and mortality. Current severity scoring systems underestimate mortality in ARDS related tuberculosis.
Though TB is treatable, individuals admitted to ICU with TB have an uncertain and desperate fate confronted with high mortality and plethora of complications, barriers to diagnosis and treatment challenges. Practice within ICU may need to change to detect and treat TB earlier and more aggressively, in order to improve outcomes. Tuberculosis in critically ill patients continues to be associated with significant mortality.5960
Funding sourceThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
The article is part of the scientific activities of the Global Tuberculosis Network (GTN).