A 68‐year‐old woman was submitted to a mediastinal lymphangioleiomyoma resection found in a follow‐up study of lower left lung resection due to bronchiectasis complicated by chylothorax. This led to a revaluation of the pulmonary specimen that revealed, in addition to inflammatory bronchiectasis, small spindle cell nodules in the lung parenchyma, similar to minute pulmonary meningothelial‐like nodules, but with smooth muscle actin immunohistochemical positivity. The possibility of initial pulmonary development of lymphangioleiomyomatosis is discussed.
Uma mulher de 68 anos foi submetida a uma ressecção de um linfangioleiomioma mediastinal observado na monitorização de uma lobectomia inferior esquerda devido a bronquiectasia, complicada por quilotórax. Isto levou a uma reavaliação do espécime pulmonar que revelou, além da bronquiectasia inflamatória, nódulos pequenos de células fusiformes no parênquima pulmonar, semelhantes a nódulos pulmonares de tipo meningotelial, mas com positividade imunohistoquímica para actina do músculo liso. A hipótese de desenvolvimento inicial de linfangioleiomiomatose pulmonar é discutida.
Lymphangioleiomyomatosis (LAM) is a rare disease of unknown aetiology, which classically occurs in women of reproductive age and, occasionally, postmenopausal age, with an estimated incidence of 1–2.6/1,000,000 women. The two most common symptoms are exertional dyspnoea and pneumothorax. Other less common symptoms include haemoptysis, nonproductive cough, chylothorax and chylous ascites.1 Pathological features consist of proliferation of immature smooth muscle cells in the airway walls, venules and lymphatics of the lung, leading to airway narrowing, obstruction and air trapping and, later on, to cystic lung lesions and cysts containing lymph fluid. Imagiology recognizes them, for the most part, in the lower lobes. The disease progresses insidiously to respiratory failure, and the patient reaches the terminal state within a variable period, ranging from a few years to about three decades.1
LAM belongs to the PEComas group and these mesenchymal tumours are composed of two cell types: epithelioid cells, usually with a perivascular location, and spindle cells, similar to smooth muscle cells, surrounding the first type with great variations in volume of the two cell types in each tumour. These cells are typically positive to melanocytic markers (HMB‐45, Melan‐A, MITF and NKI/C3) and muscle markers (α‐SMA and calponin), with less frequent observation of desmin expression. The S100 protein and cytokeratins are usually absent. At an ultra‐structural level, the cells are abundant in cytoplasmic glycogen, pre‐melanosomes, thin filaments with occasional dense bodies, hemidesmosomes and weak inter‐cellular junctions. The malignancy signs listed by the WHO for PEComas are a result of the combination of the following factors: infiltrative growth, marked hypercellularity, increased nuclear size/hyperchromasia, high mitotic activity, atypical mitotic figures and coagulative necrosis.2
LAM and tuberose sclerosis complex (TSC) may share a common genetic relationship; TSC is caused by germline mutations in tumour suppressor genes TSC1 and TSC2, located on chromosomes 9q34 and 16p13, respectively, and the tumours occur due to loss of heterozygosity in one of the genes. TSC2 gene has been implicated in the aetiology of LAM, as the mutation and loss of heterozygosity were shown in the proliferating cells. The exact pathogenesis of LAM is not yet defined, but accumulated information confirms the role of TSC2 gene somatic mutation, suggesting a spontaneous mutation in a previously normal lung cell, leading to tumour suppression dysfunction and abnormal local proliferation1,3; it was found that, when there is a deficiency of the hamartin–tuberin proteins, an anomalous activation of mTOR gives rise to uncontrolled cell growth.4 The variable expression of growth factors and their receptors in LAM may result in specific functions that facilitate the fibrogenic, proliferative and matrix regulatory actions by LAM cells.1
Another lesion that presents itself as epithelioid5 or spindle‐shaped cells with oval to indented spindle nuclei6 are minute pulmonary meningothelial‐like nodules (MPMNs); they form nests or whorls in a “Zellballen”‐like arrangement, centred on small veins, and are believed to have an oxygen‐monitoring function as a chemoreceptor, being reported as “pulmonary chemodectomas”.5 However, they have no endocrine granules and are not associated with nerves in electron microscopic analysis. Ultra‐structural studies have shown that these cells closely resemble meningothelial ones. These cells are strongly immunoreactive to the epithelial membrane antigen and vimentin, and are negative for cytokeratin, pS100, NSE and α‐SMA. They may contain a scant amount of hemosiderin, demonstrable by iron stainings, which evokes a phagocytotic‐like ability.7
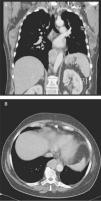
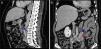
Case reportA 68‐year‐old woman with a previous history of lower left lobectomy for bronchiectasis, complicated by chylothorax (1994), underwent a follow‐up TC (2008) that showed a mass in the posterior lower right mediastinum, of 5cm diameter (Fig. 1), and was submitted to surgical resection.
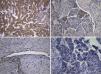
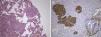
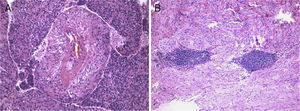
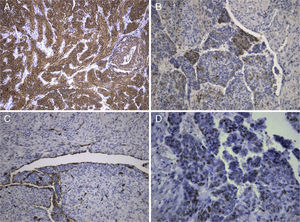
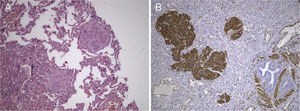
The lesion showed pink elastic tissue, with friable and congestive focal areas and, in light microscopy, showed spindle cells with monotonous oval nuclei that predominated over epithelioid cells with round and hyperchromatic nuclei; both cellular types had no mitoses or atypia. Vascularization consisted in collagenized walled vessels with eccentric lumina, from which radiated the spindle cells, and there were slit spaces scattered throughout the lesion (Fig. 2A). Rare central and peripheral lymphoid aggregates were seen, characterizing the residual lymphoid tissue of a cryptic lymph node, where they were expansive and well bounded (Fig. 2B); PAS‐diastase showed glycogen in both cells, and immunohistochemistry showed strong positivity for HHF‐35, Desmin and smooth muscle actin (Fig. 3A); multifocal positivity for HMB‐45 in epithelioid cells (Fig. 2B) and marking for CD34 in the inner lining of the cleft spaces (Fig. 2C). There were also oestrogen (Fig. 2D) and progesterone receptors, chromogranin, and NSE positivity. The mark up for low molecular weight cytokeratins, CD117 and S100 protein was negative.
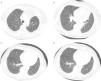
We reviewed the lower left lobectomy performed 14 years earlier, which revealed inflammatory bronchiectasis, with focal epidermoid metaplasia and an intense peri‐bronchial polymorphous inflammatory infiltrate, accompanied by bronchus‐associated lymphoid tissue (BALT), hyperplasia, and extensive abscesses continuous with the bronchial lumina. Buried in the inflammatory areas, there were nodular lesions thickening the alveolar septa (Fig. 4A), consisting of whorls of spindle cells, similar to those observed in the mediastinal mass. The immunohistochemical approach showed cytoplasmic positivity for smooth muscle actin (Fig. 4B), doubtful staining for HMB‐45, and negative for S100 protein. A hilar lymph node also showed small areas of sub‐capsular smooth muscle proliferation (Fig. 5A; B), with positive staining for HMB‐45 (Fig. 5C).
LAM is considered a primarily feminine disease, typically found in 40‐year‐old women.8 There are only four reported cases in males.1 It usually manifests itself by progressive dyspnoea or recurrent pneumothorax, chylothorax, and haemoptysis.8 In lymph nodes or thoracic duct, it leads to the formation of cystic masses that in some cases cause obstruction, leading to chylothorax or ascites.1 The definite diagnosis, determined by the ERS guidelines, requires a characteristic or compatible lung high resolution computed tomography (HRCT) and a lung biopsy with pathological criteria for LAM or a characteristic HRCT with renal angiomyolipoma, thoracic or abdominal chylous effusion, lymphangioleiomyoma/lymph node involved by LAM or definite/probable TSC.9 In the lymph nodes, LAM is characterized by trabecular smooth muscle proliferation, separated by spaces outlined by sinusoidal endothelium,10 occasionally with foci of lymphocytes scattered throughout the muscle cells and a trace of pre‐existing lymph nodes.8 In the lung encasement, the proliferation of smooth muscle cells occurs in perivascular, peribronchial and interstitial spaces, forming microscopic nodules on the walls of the peripheral airways, involving the interstitial lymphatic vessels2,10 which is often associated with cystic “emphysematous” formation. The spindle cells are smooth and muscle‐like but can take on the appearance of clear, vacuolated or hollow cells.10 Immunohistochemical expression is verified for melanocytic and muscular markers, oestrogen receptors and even for progesterone.2
Oestrogen has been considered an important factor in the progression of the disease since LAM has never been reported before menarche; it is known to accelerate during pregnancy, and remission is confirmed, in some cases, after oophorectomy. Additionally, oestrogen and progesterone receptors were identified in a sub‐population of smooth muscle cells, even when the disease affects males.1
There is no definitive treatment; although there is no sound evidence of its efficacy, progesterone is the most commonly used treatment for women with LAM; mTOR inhibitors, like Sirolimus, should be considered on a case‐to‐case analysis.9 Complications are treated symptomatically, and lung transplantation offers the only hope for pulmonary LAM cure, with a survival rate of 69% and 58% in one and two years, respectively.1
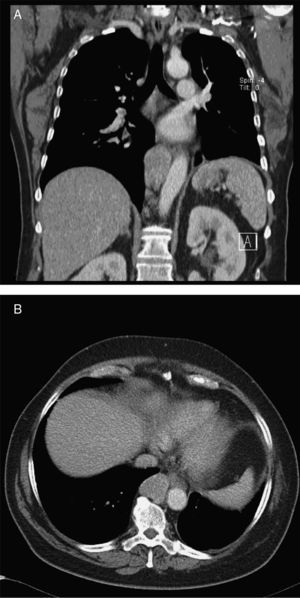
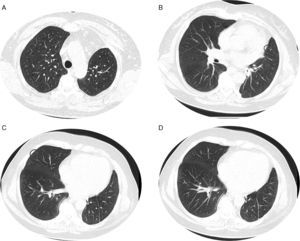
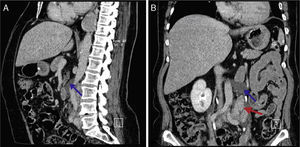
Given the morphological and immunohistochemical characteristics, the diagnosis was nodal lymphangioleiomyoma. Considering that an exclusively extra‐pulmonary involvement is rare,5 we carried out a careful study of the lobectomy, performed 14 years earlier, for bronchiectasis complicated by chylothorax. We also found a pulmonary nodular interstitial proliferation of spindle cells, which had been initially interpreted as minute pulmonary meningothelial‐like nodules (MPMNs), secondary to local hypoxaemia, but revealing muscular differentiation with positivity for smooth muscle actin, which has not been reported in this entity.7 One of the hilar lymph nodes also had small sub capsular smooth muscle areas with immunostaining for HMB‐45. There had been no typical radiological LAM aspects in the first surgery; it had only shown features compatible with bronchiectasis and pleural effusion. In 2008, despite the development of exertional dyspnoea, there were no reported findings consistent with pulmonary involvement by LAM (Fig. 6). However, assessing the images in retrospect, and facing an established pathological diagnosis of lymphangioleiomyoma, we can put a different value on a few visible lucencies, some of them surrounded by slight ground‐glass opacities. These looked like scattered lung cysts (with well defined walls, that although thin, looked too visible and thick to correspond only to emphysematous bullae, in an otherwise normal appearing lung). They were well defined, of small dimensions, similar to each other and distributed throughout the lungs. These are very slight changes, which do not allow an unequivocal diagnostic imaging of the disease, but also cannot be ignored or overlooked, and may, in fact, correspond to the initial and slight pulmonary lymphangioleiomyomatosis. Abdominal CT scan also revealed masses consistent with dilation of abdominal lymph vessels and enlarged lymph nodes (Fig. 7).
Axial sharp algorithm CT images of chest (B; D) and axial high‐resolution CT images (A; C): few visible lucencies (blue circles) of small dimensions, similar to each other and distributed throughout the lungs, well defined with a discernible wall, some of them surrounded by slight ground‐glass opacities (C).
The setting of nodal lymphangioleiomyoma raises the possibility that these pulmonary nodules with smooth muscle differentiation could be a preclinical onset of pulmonary LAM, or a subclinical pulmonary involvement in patients with extra‐pulmonary disease.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestPossible conflicts of interest for each author are disclosed.
Please cite this article as: Pontes M, Barbosa C, Coelho M, Carvalho L. Linfangioleiomiomatose pulmonar inicial provável e linfangioleiomioma mediastínico. 2014;20:101–106.