Persistence of breathlessness after recovery from SARS-CoV-2 pneumonia is frequent. Recovery from acute respiratory failure (ARF) is usually determined by normalized arterial blood gases (ABGs), but the prevalence of persistent exercise-induced desaturation (EID) and dyspnea is still unknown.
MethodsWe investigated the prevalence of EID in 70 patients with normal arterial oxygen at rest after recovery from ARF due to COVID-19 pneumonia. Patients underwent a 6-min walking test (6MWT) before discharge from hospital. We recorded dyspnea score and heart rate during 6MWT. We also investigated the possible role of lung ultrasound (LU) in predicting EID. Patients underwent a LU scan and scores for each explored area were summed to give a total LU score.
ResultsIn 30 patients (43%), oxygen desaturation was >4% during 6MWT. These patients had significantly higher dyspnea and heart rate compared to non-desaturators. LU score >8.5 was significantly able to discriminate patients with EID.
ConclusionIn SARS-CoV-2 pneumonia, ABGs at discharge cannot predict the persistence of EID, which is frequent. LU may be useful to identify patients at risk who could benefit from a rehabilitation program.
Recovery from acute respiratory failure (ARF) in patients hospitalized for SARS-CoV-2 infection (COVID-19) is generally determined by the normalization of arterial blood gases (ABGs). However, more than half of these patients still complain of breathlessness even long after discharge from hospital1 but it is not known if this is associated with oxygen desaturation during exercise. One study showed that 94% of patients with COVID-19-induced pneumonia had residual computed tomography (CT) findings after a median time from discharge of 25 days, the most frequent finding being ground glass opacities (GGO).2 A recent report showed that one-fourth of these patients at 3-month follow-up after discharge still had persistent GGO on CT-scan and a reduced diffusion capacity,3 which is associated with exercise-induced desaturation (EID). However, the ability of ABGs at rest to predict persistence of EID has not yet been investigated in this disease. Conversely, we have a lot of evidence showing that lung ultrasound (LU) findings correlate strongly with CT images.4 We aimed in this study, first, to investigate the prevalence of EID in patients otherwise showing normal gas exchange at rest after recovery from COVID-19 pneumonia, and, as a secondary outcome, to explore a possible predictive role of LU in detecting EID.
MethodsThis is a prospective cohort study enrolling consecutive patients ready to be discharged after hospitalization for COVID-19 pneumonia between April 15 and May 30, 2020. The study protocol was approved by the Hospital Ethics Committee (n. 2442 EC) and written consent was obtained from all patients for the scientific use of their clinical and physiological data, in accordance with the Helsinki Declaration. The study was conducted in two Pulmonary Rehabilitation wards dedicated to COVID-19 patients (Istituti Clinici Maugeri of Pavia and Lumezzane-Brescia, Italy). Inclusion criteria were: confirmed SARS-CoV-2 infection as detected by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from a nasopharyngeal swab; admission to our sub-acute unit for care stabilization after an acute respiratory failure (ARF) episode; and recovery from pneumonia as determined by normalization of ABGs (i.e. no need of oxygen therapy at discharge according to ABG values). Exclusion criteria were neurological or orthopedic disease (chronic or new onset), which could have compromised the execution of a walking test, and use of long-term oxygen therapy before hospitalization.
The staff operating in the two wards was adequately trained and protected with filtering facepiece class 3 (FFP3) or FFP2 masks, double non-sterile gloves, long-sleeved water-resistant gowns or protective clothing, and goggles or face shield when visiting the patients. A portable LU device available exclusively in the COVID-19 ward was used.
ProtocolPatients underwent a 6-min walking test (6MWT) and LU scan. Demographic and clinical data were also collected. The 6MWT was performed according to ATS guidelines.5 Heart rate (HR) as well dyspnea and leg fatigue and were recorded at the beginning and end of the test, using the modified BORG scale.6 LU was performed by an expert physician using a GE portable system (Logiq E) equipped with a convex transducer (3.5–5 MHz). The approach used was the standard sequence of ultrasound scans in 14 anatomic chest landmarks.7 A score from 0 to 3 was attributed for each area, and the area scores were summed to give a total LU score ranging from 0 (normal) to 42 (worst). Patients were defined as “desaturators” or “non-desaturators” depending on whether or not they showed a drop in oxygen saturation of ≥ 4% at the 6MWT.8
Statistical analysisStatistical analysis was performed using STATA 11 (StataCorp LLC) and R software (GPL, version 3.6.1, www. r-project.org). Continuous variables were expressed as mean (±Standard Deviation), and binary and categorical variables as frequencies and percentage. For comparison of categorical and binary variables, the chi-square test was used. Differences between desaturators and non-desaturators were defined using unpaired t-test.
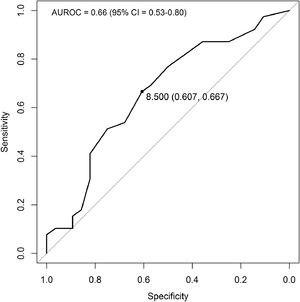
The threshold score of LU able to discriminate patients with desaturation ≥ 4% during 6MWT was identified by a bootstrap approach and using receiver operating characteristic (ROC) curves. The optimal threshold was defined as the point closest to the top-left part of the plot with perfect sensitivity or specificity.
The risk of desaturation ≥ 4% during 6MWT was explored by odds ratio (OR) analysis including anthropometric and clinical characteristics. Values of p < 0.05 were considered statistically significant.
ResultsSeventy consecutive patients admitted to our sub-acute unit after ARF due to COVID-19 pneumonia who met the enrolment criteria were included in this study. Mean age was 66.1 (±11.6) years; 71.4% were male. Demographic and clinical data are reported in Table 1. Regarding treatment for ARF in the acute ward, 57% of patients received non-invasive ventilation (NIV) while 27% required invasive mechanical ventilation (IMV). Arterial blood gases at discharge showed a mean PaO2 of 75.8 (±12.9) mmHg, and a mean PaCO2 of 34.0 (±5.0) mmHg. The mean alveolar-arterial difference of oxygen (A-aO2) was 33.4 (±15.1). Thirty of the 70 patients (43%) were desaturators and 25/30 (83%) showed a nadir SpO2 <90% during the 6MWT. Desaturators had a significantly higher length of stay (LOS) both in the acute and subacute units. Moreover, they had a significantly higher increase of dyspnea score than non-desaturators, and their HR at the end of 6MWT was significantly higher (p = 0.022). No differences were observed between desaturators and non-desaturators in terms of PaO2 and A-aO2 at rest or prevalence of comorbidities (cardiovascular, COPD, and diabetes). Frequency of NIV use and IMV did not differ between the two groups. Desaturators had a significantly higher LU score than non-desaturators (11.8±5.3 vs. 7.4±5.3, respectively). From the ROC curve (Fig. 1), the best cut-off value of LU for discriminating desaturators from non-desaturators at 6MWT was 8.5 (sensitivity = 0.67, specificity = 0.61).
Patient demographic and clinical characteristics for the whole group and according to presence or not of exercise-induced desaturation.
Variables are expressed as mean (SD) or percentage (%). ABGs, arterial blood gases; A-aO2, alveolar-arterial gradient; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HR, heart rate; IMV, invasive mechanical ventilation; LOS, length of stay; LU, lung ultrasound; NIV, non-invasive mechanical ventilation; PaO2, arterial oxygen tension; PaCO2, arterial carbon dioxide tension; SpO2, pulse oximetry; ∆, difference between start and end of 6-min walking test. p value <0.05 in bold.
LU > 8.5 significantly predicted EID [OR 4.4 (95% CI 1.6–12.7)] as well as heart rate > 120 bpm (median value) during 6MWT [OR 2.7 (95% CI 1.0–7.2] and total LOS > 40 days (median value) [OR 2.7 (95% CI 1.0–7.2].
DiscussionThe main findings of this study were: (1) more than 40% of patients with normal ABG at rest after recovery from SARS-CoV-2 pneumonia had significant EID associated with increased dyspnea and higher heart rate; (2) LU score > 8.5 was a significant predictor of EID.
Our study showed that normal values of arterial PaO2 at rest cannot predict the persistence of EID, which may be one of the most important causes of persistent effort dyspnea after recovery from COVID-19 pneumonia. A recent report on hospitalized COVID-19 patients followed up by phone or in-person 4-6 weeks after discharge showed that up to 63% of them complain of breathlessness1. To our knowledge, no one has investigated the persistence of oxygen desaturation during exercise with a standardized walking test. In our cohort of patients discharged with normal gas exchange values, almost half were desaturators at 6MWT. Moreover, the level of SpO2 reached by most of them (83%) during the 6MWT was below 90%. This means that without a standardized test we cannot predict the presence of EID simply based on ABGs. To overcome a possible bias represented by hypocapnia, still persistent in some patients and leading to a higher PaO2 value at ABGs, we considered also the A-aO2 value that did not differ between the two groups. Desaturators showed a delta dyspnea score and higher heart rate at the end of the 6MWT, but no difference in leg fatigue or total walking distance. The distribution of comorbidities, especially those that may impact on exercise desaturation (COPD and chronic cardiac diseases) between the two groups was similar, and comorbidities represented only a small percentage of the total patients. Desaturators had a longer LOS (total and in the acute phase). Probably this may be explained by a more frequent need of ventilatory support as shown by a trend, though not significant, for more frequent recourse to IMV and NIV. In other similar lung diseases, such as interstitial lung disease in the early stages, the ability of oxygen saturation at rest to predict EID has not yet been fully clarified and is still a matter of debate. In fact, the majority of studies used oxygen saturation at rest as an estimation of gas exchanges and found opposite results in terms of their ability to predict EID.9,10 However, all the studied patients had a baseline SpO2 higher than 90% and a threshold of SpO2 that could predict EID was never identified. In our study, arterial PaO2 and A-aO2 did not differ at rest between the two groups and were not predictive of EID. The only variable found to be a significant predictor of EID was the LU score. In particular, we found that a median LU score higher than 8.5 was associated with an almost 4-fold increase in the risk of EID.
LU score has already been used in previous clinical studies on SARS CoV-2 pneumonia. A high LU score was found to be associated with the severity of pneumonia.11 A median LU score higher than 24 was found to be associated to worsening, defined as a combination of high-flow oxygen support, intensive care unit admission, or 30-day mortality.12 LU can predict the persistence of EID by detecting the presence of B lines and/or consolidations in different lung areas. These sonographic alterations are an expression of an interstitial syndrome leading to delay of the alveolar-capillary oxygen diffusion, evident above all during exertion when the available time is reduced due to the increased respiratory rate. This functional alteration can also be detected with pulmonary function tests. A recent systematic review of studies that used lung function testing to assess post-infection COVID-19 patients showed a reduction of diffusion capacity in almost 40% of the studied patients.13
However, access to a pulmonary function lab was quite difficult during the pandemic.14 LU has the advantage of being available at the point of care: a suitably skilled physician can perform it in all clinical settings, and the time it takes to perform and the cost involved are negligible. Moreover, it is now becoming widely used in clinical practice from the first stages of pneumonia diagnosis. This facilitates its use also at discharge for identifying patients who may eventually need a program of pulmonary rehabilitation and/or prolonged pharmacological therapy with a closer follow-up until the clinical picture improves.
One limitation of our study is the lack of data about coexistent pulmonary thromboembolism. In fact, we were able to perform a CT pulmonary angiography only in a small percentage of cases (25%) owing to the protocol in use in our wards aimed at reducing the risk of contamination in the hospital. However, the primary outcome of this study was to explore, by means of 6MWT, the prevalence of EID in patients otherwise showing normal gas exchanges at rest. The missing information did not affect the outcome, but it may explain the reason why we did not find a very high value of sensitivity and specificity of LU in detecting EID.
ConclusionIn a group of patients admitted for pulmonary rehabilitation after ARF due to SARS-CoV-2 pneumonia and showing a complete recovery of ABGs at rest, half of them showed persistent EID associated to higher dyspnea on exertion and higher HR. A LU score higher than 8.5 was associated with a more than 4-fold higher risk of developing EID. Hence, LU could be useful for selecting patients at risk of EID to be investigated with a standardized test such as 6MWT and eventually referred for a rehabilitation program. Prospective studies are needed to confirm these data and the ability of the proposed LU threshold to predict EID at any point in the follow-up.