Short, valid and easy to use tools are needed to monitor non-invasive ventilation in clinical practice and for organization of home mechanical ventilation services. The aim of this study was to develop a professional translation and cultural adaptation of the Portuguese S3 non-invasive ventilation questionnaire.
234 stable patients (128 male patients, 53.8%) with a mean age of 69.3 years under long-term home non-invasive ventilation were recruited from a single-center outpatient clinic. The most frequent diagnostic groups were obesity hypoventilation syndrome, chronic obstructive pulmonary disease and restrictive chest wall disorders.
The Portuguese version of the questionnaire was obtained using translation back-translation process with two professional translators. Internal consistency for the total score was good (Cronbach’s α coefficient of 0.76) as well as for the “respiratory symptoms” and the “sleep and side effects” domains (Cronbach’s α coefficient=0.68 and Cronbach’s α coefficient=0.72, respectively). An exploratory factor analysis was performed leading to an explained variance of 54.6%, and resulted in 3 components.
The Portuguese version of the S3-NIV questionnaire is a simple and valid tool for the routine clinical assessment of patients receiving home NIV.
Home non-invasive ventilation (NIV) is indicated in patients with chronic respiratory failure (CRF) of different causes and its utilization in recent decades has been increasing both due to widening indications and improved health care setting organization.1,2 It is well established that not only the underlying disease, but also the intervention can have a deep impact on the patients’ health-related quality of life (HRQoL)3–5
Classical physiological variables used to monitor efficacy of NIV (such as spirometry findings and blood gas analysis) correlate poorly with reported impairment of physical function or overall health status and hence provide an incomplete picture of impaired health.6–9
Moreover, patients with CRF on NIV face unique challenges such as the dependence on external device for daily life, the number of hours spent on ventilation, limited possibilities to work/pursue daily activities, as well as subtle changes in disease progression. The impact of disease on patients' health, daily life and well-being must be measured directly from the patients themselves, by means of validated health status questionnaires.
The Severe Respiratory Insufficiency Questionnaire (SRI) is a multidimensional instrument with good psychometric properties designed to measure specific HRQoL in patients with CRF receiving home mechanical ventilation (HMV).10 It was originally developed in German and has been validated in many languages including Portuguese.11 It was developed for clinical research purposes and it is currently the most widely used HRQoL questionnaire in studies, but it is time consuming and not routinely used for clinical practice and it does not address NIV side effects which may offset some of the health benefits.
The S3-NIV questionnaire developers selected all items pertaining to “respiratory complaints” and “attendant symptoms and sleep” from the SRI questionnaire10 and items concerning comfort and side effects were obtained by qualitative interviews with patients and other comfort scales with no previous formal psychometric validation.12 After item analysis and reduction, the authors concluded the final instrument with 11 items, 5 related to respiratory symptoms, 2 related to sleep and 4 concerning side effects.
The authors considered that the S3-NIV questionnaire might be the most suitable tool currently available as it has been specifically developed for monitoring patients in routine clinical practice in NIV services but it is not intended to be a surrogate measure of general health status or quality of life.12 Specific HRQoL questionnaires, such as the SRI, therefore remain a more appropriate tool for clinical investigation.
The purpose of this study was to produce a professional translation and cultural adaptation of the S3-NIV questionnaire into Portuguese.
MethodsQuestionnaireThe S3-NIV Questionnaire is a self-administered questionnaire containing 11 items that patients score on a 5-point Likert-scale (0: always true; 1: mostly true; 2: sometimes true; 3: mostly untrue; 4: completely untrue) according to how true each statement has been for them in the 4 preceding weeks. The total score can be computed as the average of all answered items multiplied by 2.5. The lowest possible score (0) corresponds to the highest impact of disease and treatment, while the highest possible score (10) corresponds to the lowest impact of disease and treatment. The “respiratory symptoms” subscore is calculated as the average of answered items 1, 4, 5, 6 and 7 multiplied by 2.5 and the “Sleep & Side Effects” subscore is calculated as the average of answered items 2, 3, 8, 9, 10 and 11 multiplied by 2.5.
Portuguese translation and cultural adaptationThe Portuguese translation was obtained from the original French questionnaire, using the translation—back translation process by two independent professional translators.13
The equivalence of the back-translated items to the original items was evaluated and grouped into 3 categories according to previous recommendations14: category A - items that were fully equivalent; category B - items that were not fully equivalent or that contained different wording, but the content is similar; and category C - items that were not equivalent or that needed to be checked. Items rates A and B were left as they were and items rates C were reevaluated and rephrased accordingly with both of the independent translators being involved and the original questionnaire creator. The final version was written according to the New Portuguese Spelling Reform.
ValidationThis study was conducted in the Pneumology Department at Centro Hospitalar de Vila Nova de Gaia/Espinho (Portugal), a tertiary care teaching hospital. Ethical approval was obtained from the hospital Ethics Committee and written consent was obtained from all included patients.
Adult patients with CRF, from a wide variety of causes, established on HMV for at least 30 days were eligible for the study. Exclusion criteria were refusal to participate, incapacity to understand or answer the questionnaire or an exacerbation in the preceding 3 months.
Patients were categorized into six categories: chronic obstructive pulmonary disease (COPD), combined COPD and obstructive sleep apnea (COPD+OSA), restrictive chest wall disorders (RCWD), obesity hypoventilation syndrome (OHS), neuromuscular disorders (NMD), and interstitial lung disease (ILD).
Statistical analysisData are presented with mean and standard deviation or median and interquartile range. T-test was used to assess differences between two groups; comparisons between the different pathologies (with respect to age, BMI, FEV1%, FVC%, S3-NIV scales) were performed using one-way Analysis of Variance (ANOVA). Normality was assessed with the Kolmogorov–Smirnov test. If normality or homogeneity of variance assumptions were not verified, the Kruskal–Wallis (KW) test was used. Post hoc comparisons were based on Tukey’s HSD or on the Mann–Whitney (MW) test with a Bonferroni correction. Spearman Rank correlation was used to investigate the associations between different variables. Internal consistency was assessed via Cronbach’s alpha. An exploratory Factor Analysis was performed with Principal Component extraction and Varimax rotation. Statistical computations were performed with IBM SPSS Statistics for Windows, Version 25.0 (Armonk, NY: IBM Corp.). Two tailed significance assumed for p<0.05.
ResultsConsidering the translation-back translation process, all items were rated as A except item 10 rated as B for questionable wording – the first translation used the term pressure and it was considered to be too technical for patients, and so after sampling different wording with colleagues and patients, all authors and translators agreed on the simplified version “the air from the ventilator is too strong”.
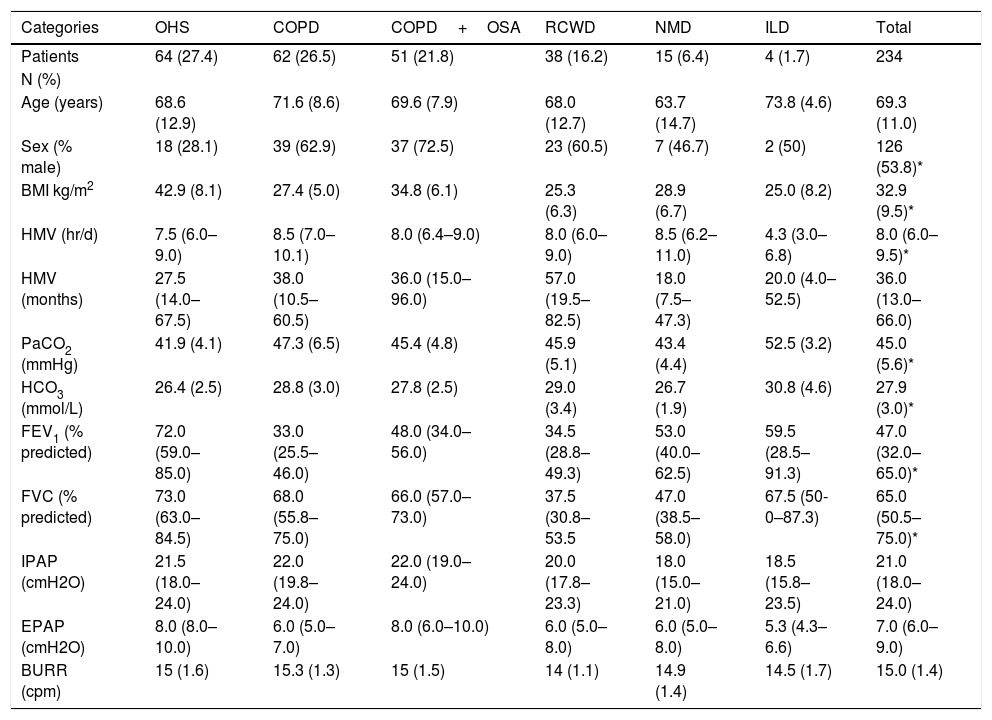
Clinical characteristics of 234 included patients are reported on Table 1.
Patients and ventilation characteristics.
| Categories | OHS | COPD | COPD+OSA | RCWD | NMD | ILD | Total |
|---|---|---|---|---|---|---|---|
| Patients | 64 (27.4) | 62 (26.5) | 51 (21.8) | 38 (16.2) | 15 (6.4) | 4 (1.7) | 234 |
| N (%) | |||||||
| Age (years) | 68.6 (12.9) | 71.6 (8.6) | 69.6 (7.9) | 68.0 (12.7) | 63.7 (14.7) | 73.8 (4.6) | 69.3 (11.0) |
| Sex (% male) | 18 (28.1) | 39 (62.9) | 37 (72.5) | 23 (60.5) | 7 (46.7) | 2 (50) | 126 (53.8)* |
| BMI kg/m2 | 42.9 (8.1) | 27.4 (5.0) | 34.8 (6.1) | 25.3 (6.3) | 28.9 (6.7) | 25.0 (8.2) | 32.9 (9.5)* |
| HMV (hr/d) | 7.5 (6.0–9.0) | 8.5 (7.0–10.1) | 8.0 (6.4–9.0) | 8.0 (6.0–9.0) | 8.5 (6.2–11.0) | 4.3 (3.0–6.8) | 8.0 (6.0–9.5)* |
| HMV (months) | 27.5 (14.0–67.5) | 38.0 (10.5–60.5) | 36.0 (15.0–96.0) | 57.0 (19.5–82.5) | 18.0 (7.5–47.3) | 20.0 (4.0–52.5) | 36.0 (13.0–66.0) |
| PaCO2 (mmHg) | 41.9 (4.1) | 47.3 (6.5) | 45.4 (4.8) | 45.9 (5.1) | 43.4 (4.4) | 52.5 (3.2) | 45.0 (5.6)* |
| HCO3 (mmol/L) | 26.4 (2.5) | 28.8 (3.0) | 27.8 (2.5) | 29.0 (3.4) | 26.7 (1.9) | 30.8 (4.6) | 27.9 (3.0)* |
| FEV1 (% predicted) | 72.0 (59.0–85.0) | 33.0 (25.5–46.0) | 48.0 (34.0–56.0) | 34.5 (28.8–49.3) | 53.0 (40.0–62.5) | 59.5 (28.5–91.3) | 47.0 (32.0–65.0)* |
| FVC (% predicted) | 73.0 (63.0–84.5) | 68.0 (55.8–75.0) | 66.0 (57.0–73.0) | 37.5 (30.8–53.5 | 47.0 (38.5–58.0) | 67.5 (50-0–87.3) | 65.0 (50.5–75.0)* |
| IPAP (cmH2O) | 21.5 (18.0–24.0) | 22.0 (19.8–24.0) | 22.0 (19.0–24.0) | 20.0 (17.8–23.3) | 18.0 (15.0–21.0) | 18.5 (15.8–23.5) | 21.0 (18.0–24.0) |
| EPAP (cmH2O) | 8.0 (8.0–10.0) | 6.0 (5.0–7.0) | 8.0 (6.0–10.0) | 6.0 (5.0–8.0) | 6.0 (5.0–8.0) | 5.3 (4.3–6.6) | 7.0 (6.0–9.0) |
| BURR (cpm) | 15 (1.6) | 15.3 (1.3) | 15 (1.5) | 14 (1.1) | 14.9 (1.4) | 14.5 (1.7) | 15.0 (1.4) |
Abbreviations: COPD, chronic obstructive pulmonary disease; OHS, obesity-hypoventilation syndrome; RCWD, restrictive chest wall disorders; COPD+OSA, combined COPD and obstructive sleep apnea; NMD, neuromuscular disorders; ILD, interstitial lung disease; BMI, body mass index; HMV, home mechanical ventilation; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; IPAP, inspiratory positive airway pressure; EPAP, expiratory positive airway pressure; BURR, back up respiratory rate.
Note: values are presented as mean and standard deviation, with the exception of months with HMV, FEV1 and FVC, which are presented as median and 25–75 quartiles.
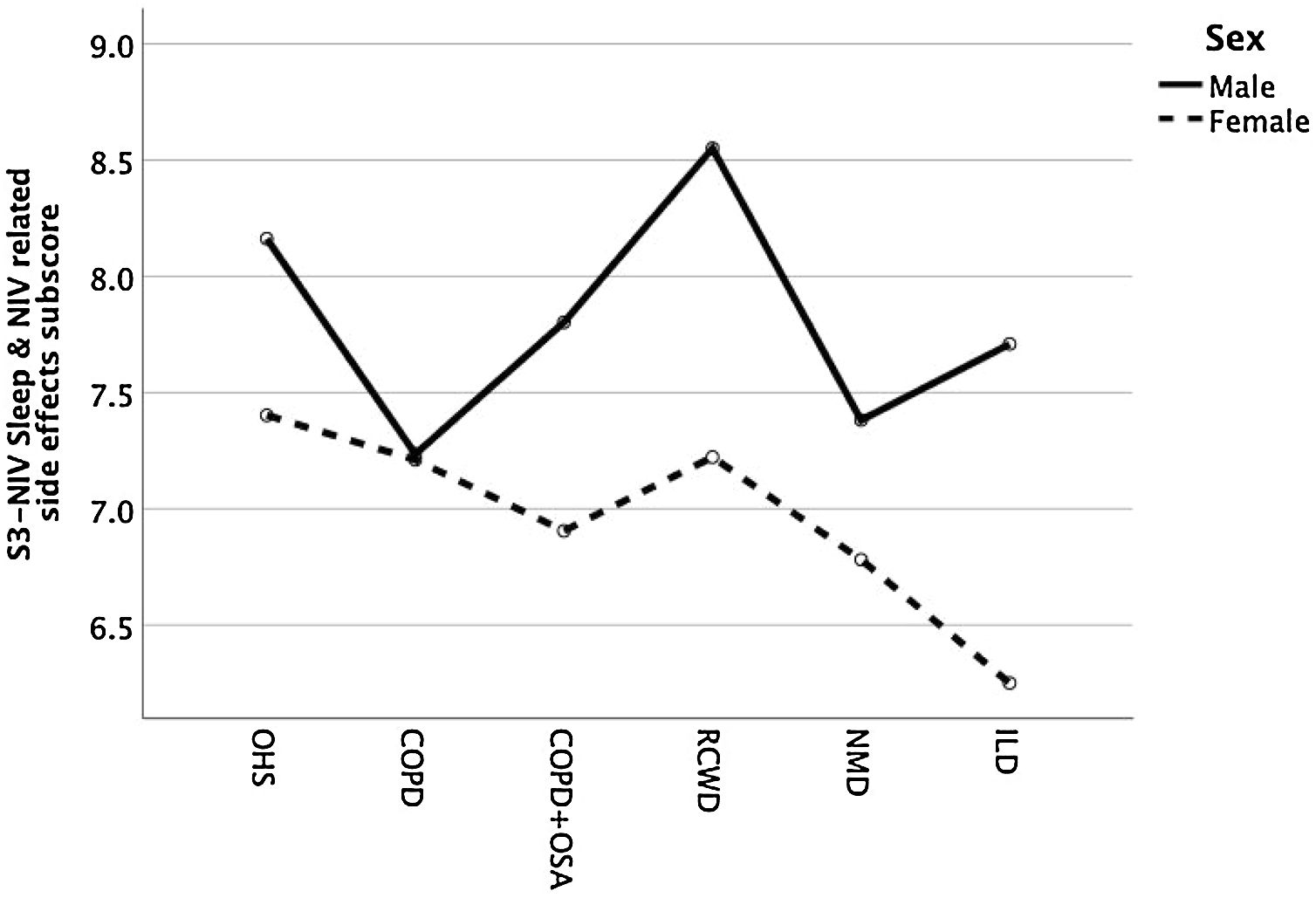
Overall, there was a slight predominance of male patients, except in NMD and most significantly in OHS patients, where almost ¾ of patients are female. The mean (Standard Deviation) age was 69.3 (11.0) with no statistical difference between different disease groups. The most common diagnostic groups were OHS and COPD (with and without associated OSA), corresponding to more than three quarters of the patients. The group of NMD patients included patients with Amyotrophic Lateral Sclerosis (6), type 1 myotonic dystrophies (3), Hereditary Myopathies with Early Respiratory Failure (3), metabolic myopathies (2) and neuroacantocitosis (1). The ILD group included idiopathic pulmonary fibrosis (2), chronic hypersensitivity pneumonitis (1) and unclassifiable ILD (1).
All patients were Portuguese native speakers.
All patients were on pressure mode ventilation, the vast majority (93.2%) on spontaneous-timed mode (median backup respiratory rate of 15) and the remainder on spontaneous mode. The most commonly used interface was oronasal mask (74.4%) and nasal mask (24.8%), with 1 patient with nasal pillows and another with tracheostomy (0.4% each). Less than on third of the patients (32.1%) were using a ventilator built-in humidifier.
Included patients were on HMV on average for 3 years, with a minimum of 3 months and a maximum of 240 months, with RCWD on longest period of time and NMD for shortest periods, although the differences are not statistically relevant.
The majority of the questionnaires were self-administered. Seventy-eight patients (33.3%) required help, because they were unable to read, had not brought their reading glasses or were physically too disabled to write (they were helped mostly by relatives). Patients took approximately 5min to complete the questionnaire.
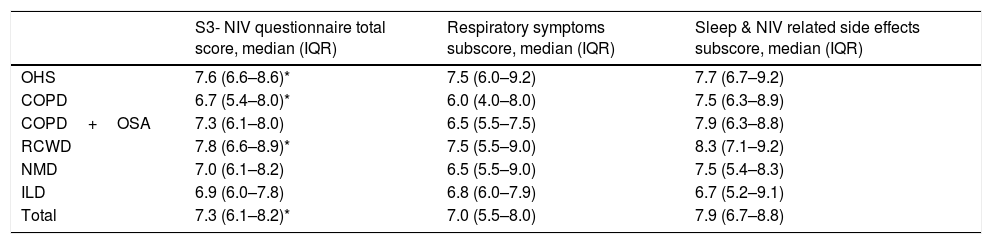
The rate of missing values on S3-NIV items was low for all items (1.7%). Data on total score and subscales are reported on Table 2.
S3NIV total and subscales’ results according to pathology groups.
| S3- NIV questionnaire total score, median (IQR) | Respiratory symptoms subscore, median (IQR) | Sleep & NIV related side effects subscore, median (IQR) | |
|---|---|---|---|
| OHS | 7.6 (6.6–8.6)* | 7.5 (6.0–9.2) | 7.7 (6.7–9.2) |
| COPD | 6.7 (5.4–8.0)* | 6.0 (4.0–8.0) | 7.5 (6.3–8.9) |
| COPD+OSA | 7.3 (6.1–8.0) | 6.5 (5.5–7.5) | 7.9 (6.3–8.8) |
| RCWD | 7.8 (6.6–8.9)* | 7.5 (5.5–9.0) | 8.3 (7.1–9.2) |
| NMD | 7.0 (6.1–8.2) | 6.5 (5.5–9.0) | 7.5 (5.4–8.3) |
| ILD | 6.9 (6.0–7.8) | 6.8 (6.0–7.9) | 6.7 (5.2–9.1) |
| Total | 7.3 (6.1–8.2)* | 7.0 (5.5–8.0) | 7.9 (6.7–8.8) |
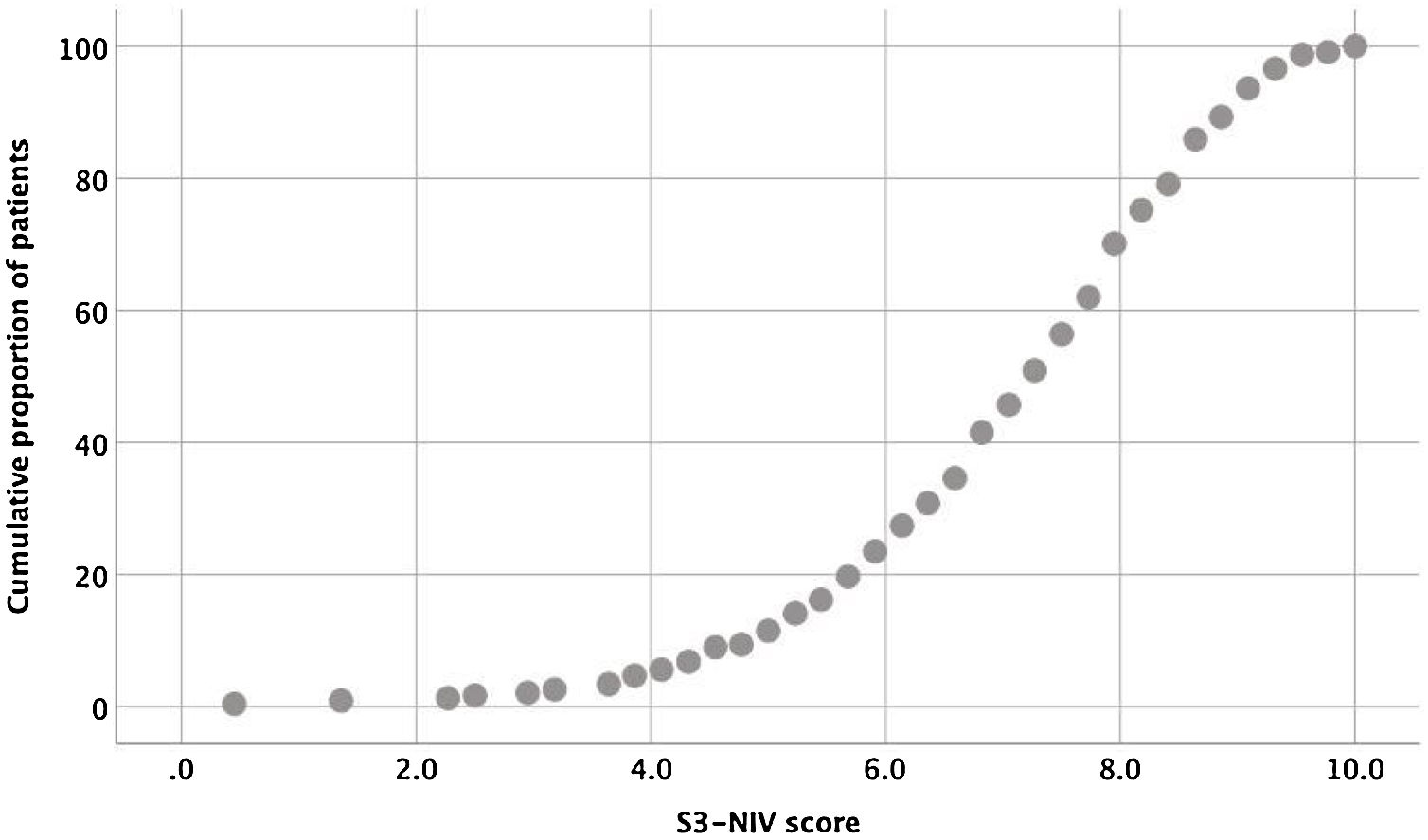
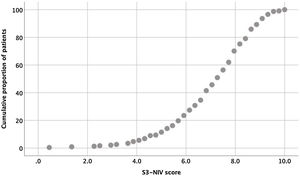
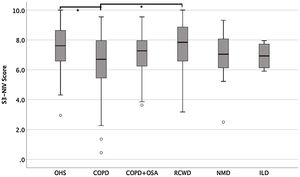
The entire scaling range was used in our validation study (minimum score of 0.5 and a maximum of 10). Of the 234 patients, 80% used 41% of the scaling range (5–9.1); 10% had a score <5.0 and 10% had a score >9.1 (Fig. 1).
When analyzing the reliability, the internal consistency of the total score was good, with a Cronbach’s α coefficient of 0.76, a Cronbach’s α coefficient of 0.72 for the “sleep & NIV-related side effects” dimension and slightly lower coefficient of 0.68 for “respiratory symptoms” dimension.
An exploratory factor analysis was carried out (data not shown) giving a Kaiser–Meyer–Olkin (KMO) of 0.80 and a significant Bartlett test to sphericity. Three factors explained 54.6% of the total variance. A varimax rotation was used and the first factor, which could be designated as daytime Dyspnea, correlated to items 1, 4, 5 and 7. The second factor, which could reflect the NIV side effects, correlated with items 8, 9, 10 and 11. The third factor that includes items 2 and 3 related breathing difficulties during sleep and headache could be perceived as sleep quality. Item 6, related to mucus production, did not correlate with any of the factors.
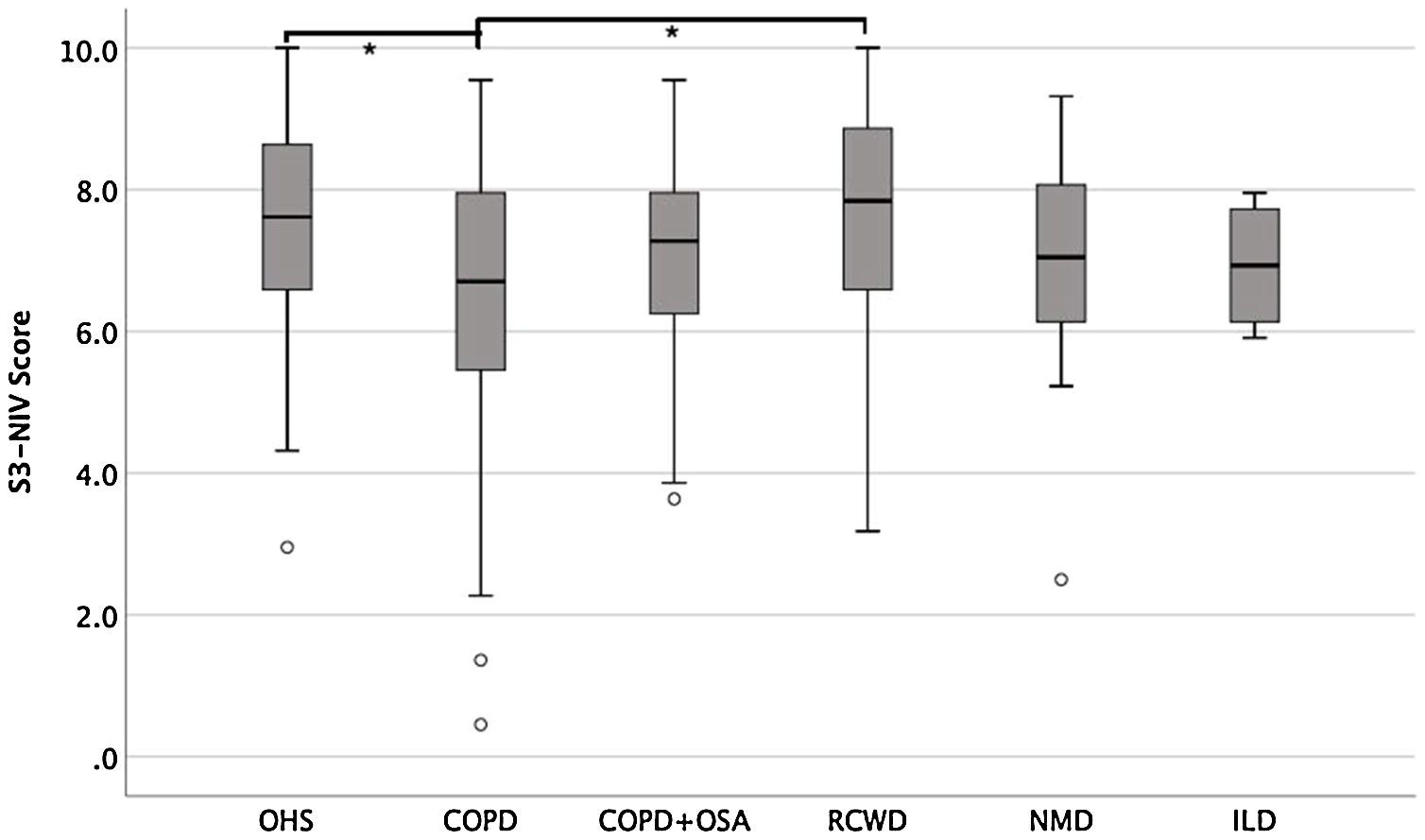
Fig. 2 shows S3-NIV total scores by disease category with no floor or ceiling effect in any disease category.
The median of the S3-NIV questionnaire score was 7.3 (IQR 6.1–8.2). Data on total and subscales scores are reported in Table 2 and stratified by disease. The impact of disease and treatment in COPD patients measured by S3-NIV score was statistically higher (lower scores) compared to OHS and RCWD patients. This difference is mostly related to the respiratory symptoms’ component of the scale.
The S3-NIV total score did not correlate with objective measures of pulmonary function (FEV1 % of predicted: rho=0.19, FVC % of predicted: rho=0.02) nor with daily ventilator usage (rho=0.06). We also found no correlation between the respiratory symptoms subscore and objective pulmonary function measurement (FEV1 % of predicted: rho=0.21, FVC % of predicted: rho=0.03) and between the sleep and side effects subscore and with daily ventilator usage (rho=0.24).
There were no differences in side effects subscores in patients with or without humidifier (7.3 vs 7.6, p=0.3), but we found that ventilation for more than 12 months had significantly higher side effects score (meaning fewer side effects) than patients being ventilated for a shorter period (7.6 vs 7.0, p=0.04).
We also found that patients with HMV for over 12 months (78.8%) had higher “Sleep & NIV related side effects” subscores than patients with HMV for less than 12 months (21.2%) [7.6 vs 7.0, p=0.04].
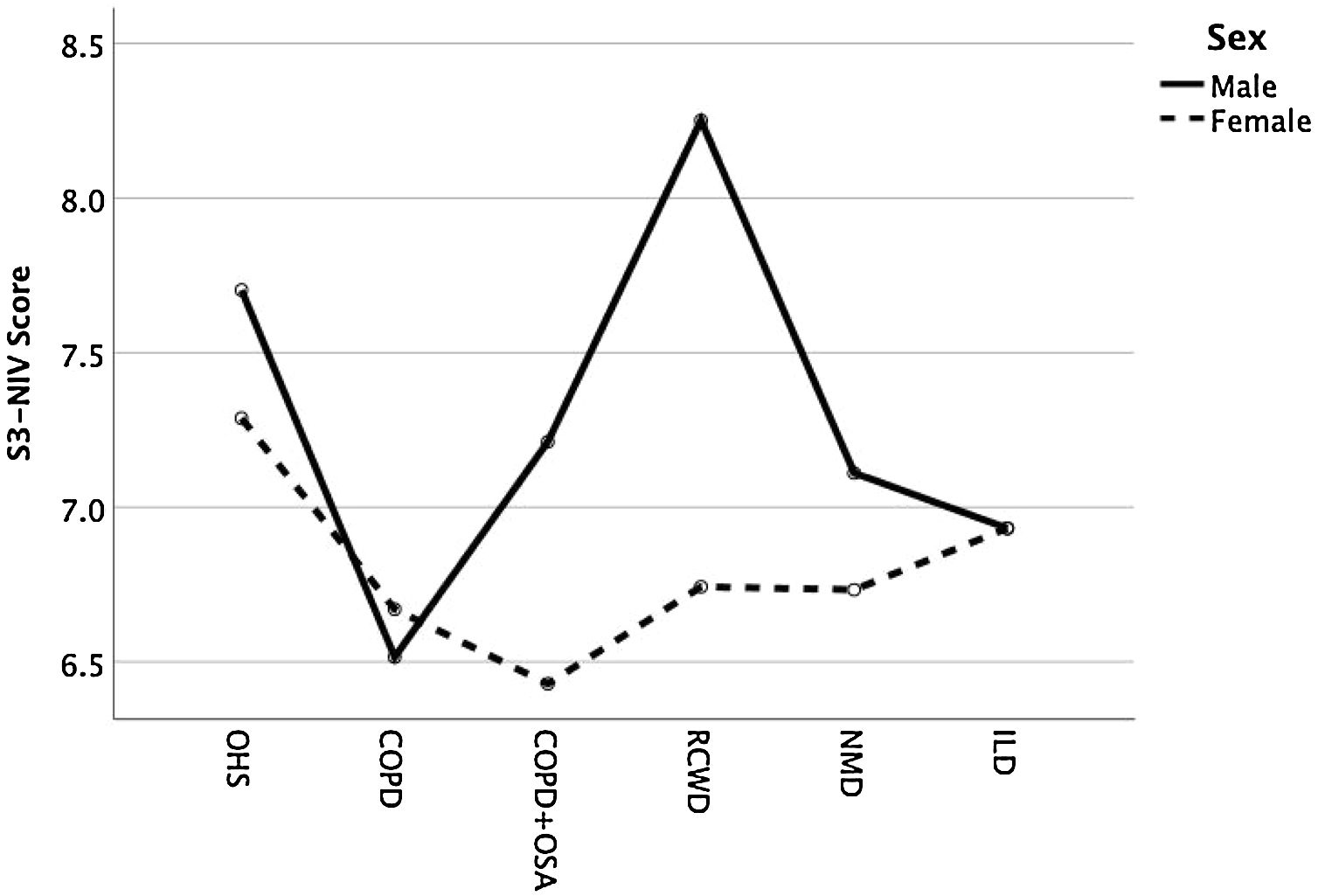
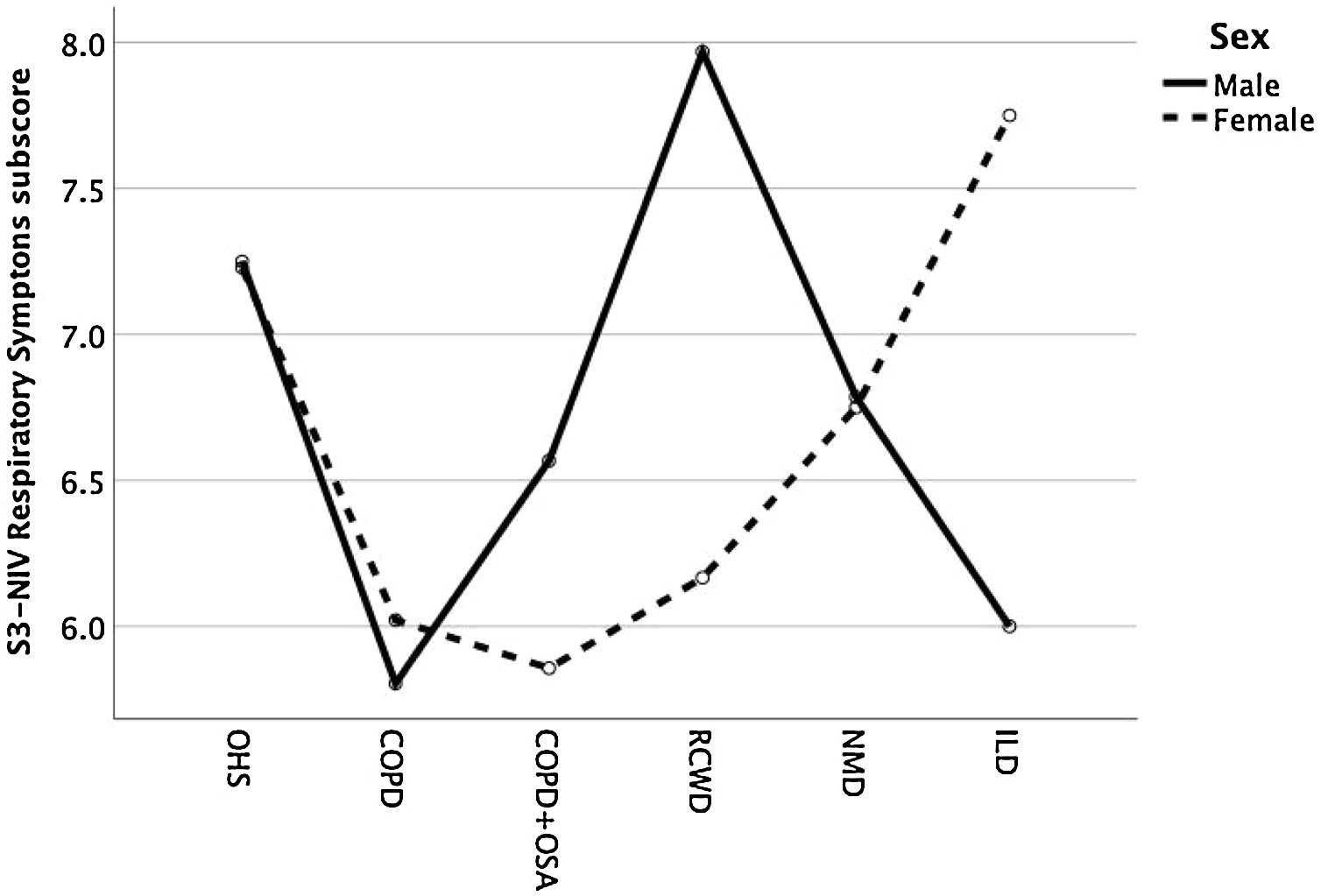
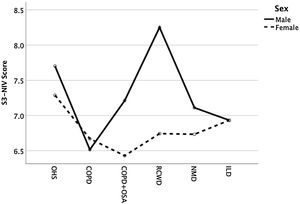
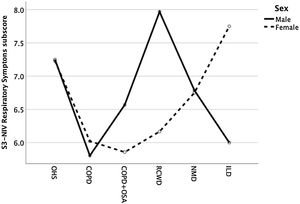
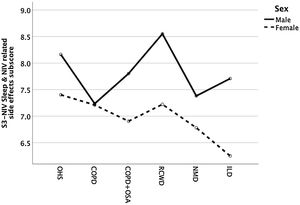
Except for COPD, we found that women had significant lower S3-NIV total scores across all disease groups. This was mainly driven by the “Sleep & NIV related side-effects” domain as illustrated in Figs. 3–5.
Patients with normocapnia (defined as pCO2<45mmHg) had better scores than patients maintaining hypercapnia (total score 7.4 vs 6.7, p=0.002; respiratory symptoms 7.0 vs 6.2, p=0.004; side effects 7.8 vs 7.2, p=0.002)
DiscussionThe S3-NIV questionnaire is a short, simple, patient-completed, specific tool that was developed to evaluate patients on home NIV in clinical practice as a complement to the monitoring of physiological variables. Although it is not formally a HRQoL questionnaire, it covers important patient centered outcomes related to NIV, i.e. respiratory symptoms, sleep quality and NIV-related side effects. This tool uses 11 items, which have been validated in a large international sample of French-speaking patients and we provide, to the best of our knowledge, the first validation, translation and cultural adaptation to a second language. Our study shows that the Portuguese version of the S3NIV, which resulted from professional translation and back-translation of the original French version, has good psychometric properties and can be used in clinical practice to monitor patients with severe CRF receiving HMV. The demand for a short, patient-oriented, self-administered tool is expected to increase greatly with the exponential development of home NIV tele-monitoring15 and possibly the widening of indications such as evaluation of noninvasive ventilation after weaning from prolonged mechanical ventilation.16
It is worth noting that, even though the New Portuguese Spelling Reform has been implemented in order to unify the writing of Portuguese between different countries, not all the countries with Portuguese as the official language have accepted it. Also, some expressions are culture-dependent and may vary significantly between countries. Therefore, this translation is essentially valid for Portugal.
Our study sample included patients with the most common diagnosis with CRF requiring HMV. Compared to the original validation study, we included a much higher percentage of COPD ventilated patients (48.3% vs 21%) which is probably related to different practices in different countries as is reported in the Eurovent study, where Portugal has one of the highest percentages of lung/airway disease patients receiving HMV in Europe.2 In our study, there was a considerably higher percentage of women (46.2% vs 25%), with similar median age (69 years) and a lower median of months on NIV (36 vs 45 months).
Our patients have a median S3-NIV score of 7.3, roughly ¾ of the scaling range and slightly higher than the original validation French-speaking cohort. With the exception of ILD patients, all the other groups have higher scores for the “sleep and side effects” dimension than the “respiratory symptoms” subscale. Although the patients have advanced diseases, this may demonstrate that patients recognize the benefits of home ventilation and have its side effects reasonably controlled, even though the majority has high inspiratory pressures.
Testing for internal consistency demonstrated acceptable to good reliability, only slightly lower than the original validation study.2
Measurements normally used as an index of functional damage or improvement (such as spirometry findings and blood gas analysis) correlate poorly with reported impairment of physical function or overall health status and hence provide an incomplete picture of impaired health.6–9 In our study, we also found a weak association between the S3-NIV total score and respiratory symptoms score and FEV1 and FVC values. This reinforces the notion that symptoms questionnaires and patient reported outcome measures must always be obtained directly from the patient and should be included in regular treatment monitoring.
There might be some potential limitations to this study. Firstly, although it is a considerable sample it represents only one center, but it represents one of only 3 highly complex multidisciplinary units in Portugal.17 Secondly, it presents cross-sectional data, as it is most common in validation questionnaires. A prospective longitudinal study will be required to assess cut off values and the minimal clinically important difference, as well as the sensitivity of this tool to changes over time or changes induced by disease progression, NIV settings or interface modifications. Thirdly, we did not incorporate an external validation with other questionnaires. From the 11 items on the scale, eight items (concerning symptoms and sleep) were selected ipsis verbis from the SRI questionnaire whose Portuguese translation has been externally validated with the SF-36 questionnaire.11 The remaining items were considered by the authors to be too different from existing questionnaires and the Quebec Sleep Questionnaire selected in the original article does not have a validated Portuguese translation and was developed to be used in obstructive sleep apnea patients.18 Therefore, the authors decided to disregard an external validation procedure.
ConclusionThis professional Portuguese translation and cultural adaptation of the S3-NIV questionnaire has good psychometric properties and it is a simple and valid tool for the routine clinical assessment of stable patients with CRF undergoing home NIV.
The Portuguese version of the S3-NIV questionnaire is available as Supplementary Fig. S1.
Conflicts of interestThe authors have no conflicts of interest to declare.