Chronic Obstructive Pulmonary Disease (COPD) is currently the 4th leading cause of death worldwide but is projected to be the 3rd leading cause of death by 2020. In Portugal, the estimated prevalence of COPD in the Lisbon region is 14.2%, and a large proportion of underdiagnosed disease has been detected.
In 2016, a Portuguese panel of experts proposed pharmacological treatment approaches to COPD based on the evidence available at the time. However, given that the GOLD 2017 report introduced considerable changes to the 2016 version, and that new evidence has emerged regarding treatment options, these proposals need to be updated. Also, and based on several studies, the concept of Pre-GOLD patients, which has diagnostic, prognostic and therapeutic implications, is introduced, along with a proposed algorithm for the identification and treatment of these patients.
Chronic Obstructive Pulmonary Disease (COPD) is currently the 4th leading cause of death worldwide but is projected to be the 3rd leading cause of death by 2020.1 In Portugal, the estimated prevalence of COPD in the Lisbon region is 14.2%, and a large proportion of underdiagnosed disease has been detected.2
In 2016, a Portuguese panel of experts proposed pharmacological treatment approaches to COPD based on the evidence available at the time.3 However, the GOLD 2017 report 4 introduced considerable changes to the 2016 version.5 According to the 2017 strategy, COPD patients continue to be separated into A, B, C or D according to symptoms and exacerbations. However, previous GOLD reports stratified patients considering three risk variables – forced expiratory volume in 1 second (FEV1), symptoms and exacerbations.5 The implications of these changes are that patients who were previously considered at risk due to poor lung function only (C or D), will now be classified as A or B, respectively, with repercussions in the therapeutic strategy recommended. The most recent GOLD 20181 did not introduce changes to the GOLD 2017 classification.4
Also, the FLAME study6–8 provided new evidence regarding treatment options for B and D patients, showing that indacaterol/glycopyrronium was more effective than salmeterol/fluticasone in reducing and preventing COPD exacerbations in patients with a history of exacerbations during the previous year, and that this efficacy was independent of different cutoffs of baseline blood eosinophilia.
Finally, and based on several studies,9–13 the concept of Pre-GOLD patients, which has diagnostic, prognostic and therapeutic implications, is introduced, along with a proposed algorithm for the identification and treatment of these patients.
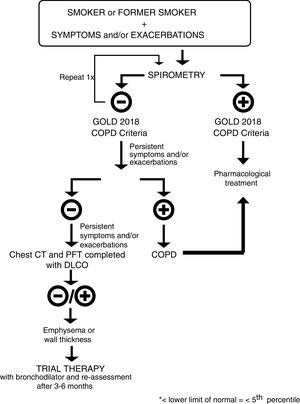
Pre-GOLD patientsThe diagnosis of COPD requires a ratio of FEV1 to forced vital capacity (FVC) of less than 0.70 as assessed by spirometry after bronchodilator use.4 However, a recent study reported that 50% of smokers with preserved pulmonary function have respiratory symptoms, including exacerbations, limitation of activity, and greater airway-wall thickening without emphysema. Among symptomatic current or former smokers, 42% used bronchodilators and 23% used inhaled glucocorticoids, without any evidence base.9 This study confirmed previous findings reporting that the effect of chronic smoking on the lungs is substantially underestimated if just spirometry is used.10 In fact, post-salbutamol FEV1 change is similar in patients with COPD and smoking controls11; a significant proportion of smokers with emphysema but without airway limitation had alterations in their quality of life, number of exacerbations and diffusing capacity of the lungs for carbon monoxide (DLCO) values.12 Taken together, all these studies confirm that FEV1 is an unreliable measure of symptom burden and exacerbations in smokers, but leave a gap on how to treat these patients in order to reduce symptoms and prevent exacerbations.13 We propose a diagnostic and treatment algorithm to evaluate and medicate these patients – Fig. 1. Every smoker or former smoker with symptoms and exacerbations should undergo spirometry. If spirometry, post-bronchodilator FEV1/FVC<0.70, values are consistent with the GOLD 2018 criteria for COPD, the patient should be treated accordingly. If the value of FEV1/FVC is between 0.6 and 0.8, spirometry should be repeated on a separate occasion,1 since this ratio may change as a result of biological variation.14,15 If the initial FEV1/FVC ratio is less than 0.6 it is very unlikely that it will rise above 0.7 spontaneously.15 If spirometry values are not in accordance with the GOLD 2018 criteria for COPD, and symptoms and exacerbations continue to persist, the patient should be checked for occult airflow obstruction using the Lower Limit of Normal (LLN) range.16 If the LLN is achieved, the patient should be diagnosed with COPD and treated according to GOLD 2018.1 If not, and symptoms and exacerbations continue to persist, a complete assessment should be done, including a thoracic Computerized Tomography (CT) scan for the differential diagnosis of emphysema, cancer and other lung pathologies. Regardless of whether the CT scan is positive or negative for emphysema/airway-wall thickening, the patient should undergo a trial with a bronchodilator and be re-assessed 3–6 months later.
This concept of pre-GOLD patients we here introduce is very similar to that recently proposed by Celli and Agusti.17 In their work, the authors argue that individuals who have symptoms similar to those of patients with COPD (namely, dyspnea, cough and/or sputum production) and structural lung abnormalities but without persistent airflow limitation may be classified as “Pre-COPDs”. The “Pre-COPDs” idea has wider implications than our pre-GOLD concept, as, according to those authors, no past or current exposure to cigarette smoke is required.
GOLD A patientsGOLD 2017/2018 clearly state that the goals of treatment for stable COPD are to reduce symptoms and risk, by improving exercise tolerance and health status and preventing disease progression, exacerbations and mortality.1,4 Results from a large recent study show that exacerbations accelerate lung function loss in subjects with established COPD, particularly when they are severe and occur in patients with mild disease.18 This is not surprising since patients with mild disease have better lung function and therefore more to lose. Also, two recent expert reviews suggest that more aggressive treatments should be implemented in the earlier stages of COPD,19 in order to slow disease progression and improve Quality of Life (QoL),20 thus obtaining the best possible outcome.19 Current data support maintenance treatment with a long-acting bronchodilator in this patient group.20 Given the above, the rationale for chronic therapy of GOLD A patients is to prevent exacerbations and slow disease progression. The GOLD 2016 report proposed short acting beta agonists (SABA) or short acting muscarinic antagonists (SAMA) as first choice therapy in these patients, with long acting beta agonists (LABA) or long acting muscarinic antagonists (LAMA) or SABA/SAMA as the alternative choice.5 Before the GOLD 2017 report was published, one expert opinion paper proposed a SABA or SAMA when post-bronchodilator FEV1/FVC<0.70 with occasional dyspnea and a LABA or LAMA when dyspnea is persistent,21 and another expert proposed the division of GOLD A patients into two sub-groups: patients with FEV1>80% and with no worsening of FEV1 in the annual assessment should be treated with a SABA or SAMA only in SOS, and patients with 50%<FEV1<80% and/or worsening of FEV1 in the annual assessment should be treated with a LABA or LAMA.3 However, according to the GOLD 2017 report,4 FEV1 should no longer guide treatment, but symptoms and exacerbation risk instead, and as such, recommendations for these patients are either a short- or a long-acting bronchodilator, which can be switched to another class of bronchodilator if there is no symptom relief, and continued or discontinued depending on symptomatic benefit. Therefore, given that the current goals of maintenance therapy are to reduce symptoms and prevent exacerbations, then a LABA should be chosen for the former (symptoms reduction),22 and a LAMA for the latter (exacerbations prevention) since it has been reported to be superior to LABA regarding exacerbation prevention.23,24 Dual bronchodilation with LABA/LAMA should not be offered to these patients because they are neither frequent exacerbators nor very symptomatic.
It is worth noting that, depending on the instrument used for the classification of symptoms, the modified Medical Research Council scale (mMRC) or the COPD assessment test (CAT), a patient may be classified as A or B 25,26 and therefore both instruments are recommended.
We propose that chronic maintenance therapy with a LABA or a LAMA should be offered to GOLD A patients. We agree with GOLD 2018 in that a switch to another class of bronchodilator can be made if there is no symptom relief, and therapy should be continued or discontinued depending on symptomatic benefit.
GOLD B patientsAlthough inhaled corticosteroids (ICS) are not recommended as maintenance therapy for GOLD B patients,3,5,21,27 real-world studies or baseline characteristics of patients enrolled in RCTs show that up to 51.8% of these patients are still medicated with ICS.28–33 In a paper from 2016, we speculated that this was mainly due to the generalized idea that a patient taking ICS will be more controlled than a patient who is not on ICS therapy, and will not exacerbate or deteriorate, which is not true.3 Our speculation is now supported by evidence from the FLAME study, that randomized a total of 1680 patients to the indacaterol/glycopyrronium 110/50μg once-daily group and 1682 to the salmeterol/fluticasone 50/500μg twice-daily group, and showed that for GOLD B patients with a history of at least one exacerbation during the previous year, indacaterol/glycopyrronium was more effective than salmeterol/fluticasone in preventing COPD exacerbations, irrespective of prior ICS/LABA/LAMA therapy,7 and was associated with no detectable increase in adverse events.6 The FLAME study also supports our previous recommendation that B patients without exacerbations, who are overtreated with ICS, should be withdrawn from ICS,3 and further suggests that even B patients with at least one exacerbation during the previous year can and should be withdrawn from ICS.6,7 Symptomatic patients are more likely to experience exacerbations34 (the ECLIPSE study showed that 52% of these patients are exacerbators),35 and therefore it is of the utmost importance to control symptoms, namely with dual bronchodilation,3,21,27 which is now clearly supported by the FLAME study and other studies from the IGNITE clinical development program for indacaterol/glycopyrronium.6,7,36,37 In the Salford Lung Study, a large randomized open-label trial designed to mimic real-world conditions, a once-daily treatment regimen of combined fluticasone furoate/vilanterol 100/25μg was compared to usual care, and the authors concluded that the fluticasone furoate/vilanterol combination was associated with a lower rate of exacerbations than usual care, without a greater risk of serious adverse events.38 It is our opinion that this study does not contradict the FLAME study for several reasons: in the Salford Lung Study38 22% of the patients included had a diagnosis of asthma, the comparator arm was usual care as determined by the general practitioner, which resulted in a variety of monotherapies and/or combination therapies (88% of patients in the usual care group were receiving an ICS-containing regimen), and unilateral crossover was permitted. Therefore, the conclusions drawn by the authors have several possible sources of bias and it is not possible to conclude from these data whether fluticasone and vilanterol is more effective than a LABA/LAMA at reducing exacerbations.
The ECLIPSE35 and FLAME6,7 studies grouped patients according to the GOLD 2016 report5 thus including FEV1 as a risk criterion. From GOLD 2017 onwards1,4 FEV1 is no longer a criterion for risk, only symptoms and exacerbations. The FLAME study states that 19.3% of patients had ≥2 exacerbations, and these will now be the GOLD D patients; as for the remaining patients involved in the study, those with ≤1 exacerbation without hospitalization, will be now classified as GOLD B. In fact, since the GOLD 2017 classification,1,4 it is to be expected that many patients formerly classified as GOLD D would now be classified as GOLD B, because they were classified as GOLD D due to FEV1. These GOLD B patients are the most heterogeneous and unstable, and may eventually exacerbate.35 Thus, the conclusions of the FLAME study hold true regardless of the GOLD stratification.
However, with this new classification, and as many former GOLD D patients will now be considered GOLD B, they are probably on ICS therapy, as recommended for GOLD D patients. These patients, previously classified as GOLD D due to FEV1 and not due to exacerbations, should be withdrawn from ICS.
In a previous paper we proposed that GOLD B patients should be divided in two subgroups, BX1 and BX2, and the therapeutic approach should be based on this subdivision.3 Since GOLD 2017 led to changes in COPD patient classification, in this paper we have adapted, our proposal as shown in Table 1.
proposed division of GOLD B patients in two subgroups and respective therapeutic approaches.
| Sub-group characteristics | Therapeutic approach |
|---|---|
| BX1: mMRC=2 AND 0 exacerbations; AND no cardiovascular co-morbidities | (a) if not medicated, initiate LABA or LAMA |
| BX2: mMRC>2 OR 1 exacerbation without hospitalization OR with cardiovascular co-morbidities | LABA+LAMA (“hit hard” approach) |
mMRC – modified Medical Research Council dyspnea scale; LABA – long acting β2-agonist; LAMA – long-acting muscarinic antagonist.
We propose that ICS should not be given to GOLD B patients as maintenance therapy. We further propose that previously GOLD D patients now considered GOLD B post GOLD 2017 and who are on ICS therapy should be withdrawn from it, and be monitored 3–6 months later.3
GOLD C patientsAccording to the 2017/2018 GOLD C new classification,1,4 the groups C1 (high risk due to poor lung function) and C3 (high risk due to both poor function and exacerbations)3 no longer exist and all GOLD C patients will be classified as high risk based only on exacerbations. Therefore, the main therapeutic goal will be to reduce the risk of exacerbations. As initial therapy should consist of a single long acting bronchodilator, a LAMA should be preferred to a LABA since it has been reported to be better at preventing exacerbations.23,24
We agree with GOLD 2018 that these patients should start therapy with just LAMA and then, if exacerbations continue, should preferably be switched to LABA/LAMA, instead of LABA/ICS, although the latter remains an option.
GOLD D patientsHow to start therapyMany guidelines have recommended LABA/ICS and/or LAMA as first-line therapy for higher risk patients.3,5 However, some discrepancies exist, and the therapy approach to these patients is not straightforward.3,21,27,33 A meta-analysis from 2015 concluded that LAMA/LABA seemed to be a better option for treating GOLD D patients (as defined pre-GOLD 2017/2018) than LABA/ICS.39 The FLAME study supports this conclusion, favoring indacaterol/glycopyrronium versus salmeterol/fluticasone in preventing exacerbations in GOLD D patients (as defined pre-GOLD 2017/2018),6 including patients with prior triple therapy.7 When compared with salmeterol/fluticasone, indacaterol/glycopyrronium was better at preventing all exacerbations and delaying the time to first exacerbation, any exacerbation (p<0.001), moderate-to-severe exacerbations (p<0.001) and severe exacerbations (p=0.046). The improvement over time in the total score on the St. George's Respiratory Questionnaire for COPD patients (SGRQ-C) was significantly greater than salmeterol/fluticasone after day 85. Also, at week 52, the percentage of patients who had a clinically important decrease of at least 4 points in the total score on the SGRQ-C was significantly higher and the use of rescue medication was significantly improved for the patients in the indacaterol/glycopyrronium treatment arm versus those in the salmeterol/fluticasone arm. On subgroup analysis, the advantage of indacaterol/glycopyrronium was particularly relevant for current smokers, patients with severe airflow limitation, GOLD D patients, patients with 1 exacerbation in the previous year, and previous use of LABA or LAMA. As for adverse events, indacaterol/glycopyrronium was not associated with a detectable increase in adverse events and the incidence of pneumonia was significantly lower in the indacaterol/glycopyrronium group than in the salmeterol/fluticasone group (3.2% vs 4.8%, p=0.02). This was also the first study to prospectively analyze the relevance of blood eosinophilia in COPD, and the results were similar for blood eosinophilia<2% compared to ≥2%. Other analyses in subgroups defined according to different cutoffs of baseline blood eosinophil counts provided similar results.6,8
Data from the first head-to-head study (TRIBUTE) comparing a triple combination of LABA/LAMA/ICS (beclometasone/formoterol/glycopyrronium, 100/6/12.5μg, two inhalations twice-daily) with a dual combination of LABA/LAMA (IND/GLY) have just recently been published.40 The TRIBUTE study enrolled symptomatic patients (CAT≥10) with a FEV1<50% and a history of at least one documented moderate or severe exacerbation in the past year. The primary endpoint analysis showed a 15% reduction in the rate of moderate to severe exacerbations favoring the triple combination when compared with the dual combination (p=0.043). It should be noted that no significant difference between treatment regimens was observed when moderate and severe exacerbations were analyzed separately (p=0.118 and p=0.189, respectively) or for the time to first moderate or severe exacerbation (p=0.219) and time to first severe exacerbation (p=0.405). In addition, the pre-specified subgroup analyses suggest that patients with: FEV1<30%, or emphysema or a mixed phenotype, or >1 exacerbation in the previous 12 months, or lower blood eosinophils (<2% or <200cells/μL) may not derive any benefit from LABA/LAMA/ICS compared with LABA/LAMA. Interestingly, the incidence of pneumonia was similar between the two regimens. The results from TRIBUTE are entirely in line with the current GOLD recommendations and indicate that patients with chronic bronchitis and elevated blood eosinophil counts will be more likely to benefit from a triple combination regimen. Recent data from the IMPACT study also appear to be in line with the GOLD recommendations with triple therapy with fluticasone furoate/umeclidinium/vilanterol showing benefits in patients with frequent exacerbations and in those with a severe exacerbation in the 12 months previous to study enrollment when compared with fluticasone furoate/vilanterol and umeclidinium/vilanterol.41 Despite this, and as mentioned by Suissa & Drazen, in an Editorial on the IMPACT study42: “However, the selected trial patients, most of whom were already treated with inhaled glucocorticoids and some of whom had a history of asthma, were not the natural population in which to study this question, potentially artificially inflating the observed effectiveness of the triple-therapy inhaler over dual bronchodilator treatment”. Consequently, the patient population involved in the IMPACT study makes it very difficult to interpret these data and come to a clear conclusion about its relevance for the management of COPD patients.
In face of the results described above, we recommend that GOLD D patients should start therapy with dual bronchodilation, and ICS should only be used as an add-on if patients have further exacerbations. We also propose that ICS should not be given to these patients as first line maintenance therapy.
If and when to add ICSA recent post hoc analysis of the WISDOM study concluded that a history of ≥2 exacerbations per year plus an eosinophil count ≥300cells/μL identifies individuals at increased risk of exacerbation when ICS is discontinued. These authors report that the most consistent and greatest effect was seen in patients with ≥2 exacerbations and ≥400cells/μL. Nevertheless, the authors recognize that, given the relatively small sample sizes, more studies are needed, along with prospective confirmation of the validity of the proposed subgroups of ICS-responsive individuals.43 New data on this subject have now been provided by the SUNSET study where non-frequently exacerbating patients on long-term triple therapy with tiotropium plus salmeterol/fluticasone were randomized to either continue their triple therapy regimen or switch to indacaterol/glicopirronium.44 The switch to indacaterol/glycopirronium led to a small reduction in lung function (-26mL in trough FEV1) but no difference in COPD exacerbations between treatment groups. In addition, the study also demonstrates that patients with higher blood eosinophil counts of ≥300cells/μL are more likely to benefit from triple therapy as far as loss of lung function is concerned.44
Due to the lack of more conclusive data, the recommended treatment approach is the one proposed previously.3,21,27
ConclusionsBased on the GOLD 2017/2018 and on new evidence that has emerged regarding treatment options with the FLAME study, this paper provides an update on a previous proposal for pharmacological treatment approaches to COPD patients. Also, the concept of Pre-GOLD patients, which has diagnostic, prognostic and therapeutic implications, is introduced, along with a proposed algorithm for the identification and treatment of these patients.
Financial supportFunding for this paper was provided by Novartis Portugal. Funding was used to access all necessary scientific bibliography and cover meetings expenses. Novartis Portugal had no role in the collection, analysis and interpretation of data, in the writing of the paper and in the decision to submit the paper for publication.
Conflicts of interestNuno Pires reports personal fees from Novartis. Paula Pinto reports personal fees from Novartis. Nelson Marçal reports personal fees from Novartis, Teva and Boehringer-Ingelheim. António Jorge Ferreira reports personal fees from Novartis, Bial, Boehringer-Ingelheim, GSK, Tecninfar and Teva. Cidália Rodrigues reports personal fees from Novartis. Cristina Bárbara has nothing to disclose.