Thermo-humidified nasal high flow (NHF) oxygen therapy is increasingly used in the management of respiratory failure. This therapy has recently gained attention as an alternative non-invasive respiratory support in several clinical scenarios, including acute and chronic settings. NHF enhances the patient’s comfort and tolerance when compared with standard oxygen by supplying a heated and humidified mixture of air and oxygen at flows up to 60L/min. It can be delivered through different devices. Although few studies have compared the clinical effects of different NHF systems, the purpose of this paper is to describe the major benefits of NHF and to provide a quick guide on how to implement this therapy in daily practice. We have also included a brief description of the most frequently used NHF systems.
A 56-year-old man with SARS-COV2 infection was admitted to our hospital on November 1st, after 7 days of fever. Comorbidities comprised hypertension and obesity. On the third day after admission, he was transferred to our Respiratory Intensive Care Unit due to worsening of his respiratory condition, including notable shortness of breath with minimal exertion. On arrival, the patient was alert, haemodynamically stable. His respiratory rate was 25/min with a reservoir oxygen mask at 15L/min. Blood gas analysis (ABG) revealed a severe hypoxemia and alkalosis respiratory (pH 7.51, PaCO2 35mmHg, PaO2 118mmHg, HCO3− 27mmol/L, PaO2/FiO2 148). Due to patient’s gas exchange impairment and clinical progression, NHF therapy trial was attempted.
Initial temperature, inspired oxygen fraction (FiO2) and flow rate were 34°C, 60L/min and 60% respectively.
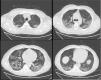
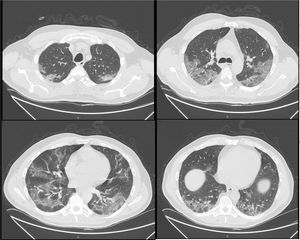
In order to reduce bio-aerosol dispersion, a surgical mask was placed over the patient’s mouth and nose.1Fig. 1 shows bilateral consolidations with ground-glass areas, mainly in the posterior dependent zones on CT-scan. Based on this radiological picture, we continued NHF in awake prone positioning. Fig. 2 illustrates oxygen pulse saturation/FiO2 (SpO2/FiO2), oxygen blood pressure/FiO2 (PaO2/FiO2) ratios and ROX index trends within the first four days of treatment. ROX index is defined as the ratio of pulse oximetry/fraction of inspired oxygen to respiratory rate (RR). This index, described by Roca et al.,2 identifies patients at risk of NHF failure. In particular, a ROX index of 4,88 is associated with a high risk of intubation. In conclusion, NHF setting was adjusted according to the maximum patient’s comfort and the best effects in terms of distending pressure and FiO2 stability. FiO2 was also targeted to achieve oxygen saturation between 94–96%. Prone positioning was implemented because we felt that the mild distension pressure generated by NHF could be enhanced by placing the ventilated areas in a dependent position, leading to increased V/Q mismatch.3
Thermo-humidified nasal high flow (NHF) oxygen therapy is increasingly used in the management of respiratory failure. This therapy has recently gained attention as an alternative non-invasive respiratory support in several clinical scenarios, acute and chronic,4 in order to enhance patient comfort and tolerance compared with standard oxygen by supplying heat and humidified mixture of air and oxygen at flows up to 60L/minutes.5
NHF can be delivered through different devices. Although few studies have compared the clinical effects of different NHF systems, the purpose of this paper is to describe the major benefits of NHF and to provide a quick guide on how to implement this therapy in daily practice. We have also included a brief description of the most frequently used NHF systems: BioRespira® (IBD), Airvo 2® and OptiFlow® (Fisher and Paykel), TNI softFlow 50® (Masimo) and Precision Flow® (Vapotherm).
Physiological benefits of NHFHigh flows air/oxygen mixture can be beneficial for both chronic and acute patients, who need additional oxygen supply:
- -
Matches patient’s inspiratory flow (stable FiO2). Conventional oxygen therapy devices such as Venturi masks or reservoir masks have holes to prevent carbon dioxide rebreathing. The patient inspires the delivered oxygen, but part of the air can be rebreathed. In addition, if the patient’s ventilatory demand exceeds the flows provided by the device, the patient will breathe part of atmospheric air. For this reason, because of the relationship with the patient-breathing pattern, FiO2 can be much lower than predicted. Pisani et al.4 observed that calculated FiO2 approached the prescribed FiO2 when the delivered gas flow rates were greater than the patient’s peak inspiratory flow rate. This means that, with low-flow systems, as the peak inspiratory demand increase, we cannot accurately estimate the FiO2 that the patient is breathing. NHF delivers a flow that is much higher than the patient’s spontaneous inspiratory flow in any condition, so actual FiO2 is close to the predicted FiO2.
- -
Improves lung mucociliary clearance. In normal conditions, inhaled air is conditioned in the nasal airways. The air is warmed and humidified by the nasal mucosa because of its highly vascular sinusoid tissue. Humidification is always recommended for flow rates above 4L/min, because the humidification function of the nasal mucosa could be insufficient. In addition, unwarmed and dry gas leads to a poor tolerance of oxygen therapy.
- -
Washes out anatomical dead space and carbon dioxide (CO2), allowing a higher fraction of minute ventilation to participate in gas exchange. Additionally, the nasal cannula, used for NHF, is the only respiratory interface that does not increase the instrumental dead space. With an oxygen mask, especially at low flow, carbon dioxide is rebreathed. NIV interfaces also increase the dead space. As a consequence, to maintain PaCO2 (and alveolar ventilation), the minute volume has to increase, either through respiratory rate or through tidal volume (VT), or even both. For these reasons, although NHF does not provide an active inspiratory support, it may improve the alveolar ventilation and decrease respiratory rate (RR).6 Fraser et al.7 found that NHF leads to a reduction in respiratory rate of 10%, and in some patients, this reduction was associated with a reduction in hypercapnia.
- -
Provides a mild distending pressure. Although NHF is an open system, high flow from the nasal cannula increases the airway pressure usually around 3cmH2O,8 depending on the flow level and the nasal prong size in relation to the nostrils. Richie et al.6 conclude that NHF could deliver a clinically relevant mean positive airway pressure directly proportional to delivered gas flow rates. In addition, high flows could act as a resistance to exhalation when the mouth is closed.9,10 However, it remains unclear whether NHF is sufficient to increase lung volume or recruit the collapsed alveoli. Evaluating end-expiratory lung volume (EELV), Corley et al.11 reported increased end-expiratory lung impedance, suggesting increasing volumes and functional residual capacity with NHF compared with low-flow oxygen therapy. Also using electrical lung impedance tomography on healthy subjects in supine and prone position, Riera et al.12 found that NHF increased global EELV, suggesting an increase in functional residual capacity, regardless of the body position. This effect could be important in patients with a higher body mass index.
- -
Attenuates inspiratory resistance and increases expiratory resistance. The attenuation of inspiratory resistance takes place essentially by providing an adequate warmed and humidified flow. It is known that in normal subjects,13 inhalation of cold air causes the activation of specific receptors or osmoreceptors in the nasal mucosa, which may induce bronchoconstriction through a mechanism mediated by the activation of muscarinic receptors.14 Besides, NHF is able to increase the expiratory resistance depending on the size of the nasal cannula and the patient’s expiratory flow.15
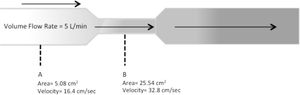
A typical NHF system consists of a flow generator, active heated humidifier, single-limb heated circuit, and nasal cannula (or tracheostomy interface). There are three types of flow generators: air-oxygen blender devices, built-in flow generators (turbines), and entrainment systems (Venturi Systems). The humidifier can be a traditional “pass-over heated humidifying system” or a “filter-type humidifying system” in which humidification is generated by passing gas through a bundle of narrow tubes, or which requires an evaporative surface in contact with the gas. A heated-wire inspiratory circuit is preferred to ensure delivery of adequately humidified medical gas as well as to avoid circuit vapour loss and condensation. The lower the ambient temperature, the more likely there is to be condensation. One major reason for the patient’s discomfort or intolerance is the interface. Beyond the circuit, condensation may also accumulate in the nasal prongs, which results in water droplet spray into the nostrils. NHF interfaces are specifically for this purpose. They can vary from a slender nasal cannula similar in appearance to a regular nasal oxygen cannula in which both the internal diameter and nasal prong bore are narrow, to a large bore nasal prong. Using slender nasal cannulas results in high flow out of the nasal prongs. The volumetric flow is the volume of gas that passes in a certain unit of time. The speed at which the volume moves is the velocity. The speed, at a constant volumetric flow, varies inversely proportional to the cross-sectional area of the tube. This is the reason why the smaller the cross-sectional area of the nasal cannula, the greater the speed (Fig. 3).16
Implementation technique- a)
General considerations (regardless of the NHF device used)
- -
Gas connection to the device (O2, and compressed air if required).
- -
Place distilled water in the humidifier.
- -
Connect the circuit, appropriate to the system used.
- -
Switch on and heat up the water until the temperature reaches 31−37°C.
- -
Select the size of the nasal cannula properly. When using a large bore nasal prong, it must not occlude more than 50% of the nostril.
- -
Position the cannula correctly in the patient’s nostrils, to avoid lateral leakage.
- -
Program the parameters, according to the equipment used (temperature, flow, and FiO2 if the setting is available).
- -
Switch on the device (some devices allow parameters to be programmed once the machine is on).
- b)
General monitoring
- -
Patients must be spontaneously breathing, alert and must be able to protect the airway.
- -
Check that there is no accumulation of condensed water in the tube, ensuring its inclination towards the heater.
- -
Aspiration of patient’s secretions, if needed.
- -
Check that the cannula is not occluded by a mucus plug or against the patient’s nose.
- -
Make sure the patient is in a semi-seated position at 45°. NHF can be implemented in “awake prone position”, if needed.
- -
Check that the device is not disconnected from the electrical source when using high-flow devices, because not many have an internal battery.
- c)
General set up
- -
Temperature: it must be targeted according to the patient’s comfort. The temperature needs to be set at higher values at cold weathers or high flows.
- -
Flow: the maximum benefit of this treatment is achieved at higher flows, although the flow should also be titrated according to the patient's comfort. It is suggested starting with flows close to 50L/m, according to the patient's tolerance, in order to increase or decrease the flow.
- -
FiO2: depending on the NHF device used, FiO2 can be a parameter to be programmed or a parameter that depends on the flow. By using an air-oxygen blender device, oxygen concentrations are stable. On the other hand, by using turbine devices, oxygen is supplied via a low-pressure system, while the device just monitors the oxygen concentration (FiO2). Any rise in the flow, will decrease the FiO2, and vice versa. Venturi high flow systems use a flow generator to create a high flow by Venturi. It is composed of a flow meter and an oxygen concentration monitor. Regardless of the system used, in all cases, FiO2 titration should depend on the SpO2 target, recommending an SpO2 range between 94% and 96%, or between 88%–92% in hypercapnic COPD patients.
Table 1 summarizes the main characteristics associated with the currently available devices.
Main characteristics of most frequent used NHF devices.
| BioRespira® IBD | Airvo2 (F&P®) | Optiflow (F&P®) | TNI softFlow 50 Masimo | Precision Flow Vapotherm® | |
|---|---|---|---|---|---|
| FLOW (L/M) | 5−120 (NHF AND CPAP USE) | 10−60 (5L/M STEPS) | 30- 60 | 1060 (0,5 STEPS) | 5−40 (1L/M STEPS) |
| FLOW GENERATION | BLOWER | BLOWER | BLENDER | BLOWER | ELECTRONIC BLENDER |
| FIO2 INDEPENDENT OF SETUP FLOW | YES | NO | YES | NO | YES |
| POSSIBILITY OF FIO2 100% | YES | NO | YES | NO | YES |
| TYPE OF CONNECTION O2 | HIGH PRESSURE SYSTEM O2 LOW PRESSURE SYSTEM O2 | LOW PRESSURE O2 (UP TO 30L/M) | HIGH PRESSURE SYSTEM AIR AND O2 | LOW PRESSURE SYSTEM O2 (UP TO 60L/M) | HIGH PRESSURE SYSTEM AIR AND O2 |
| NEED FOR COMPRESSED AIR FROM THE WALL | NO | NO | YES | NO | YES |
| BUILT-IN HUMMIDIFIER | EXTERNAL GENERIC | INCORPOATED F&P | EXTERNAL F&P | INCORPOATED INTERSURGICAL® | INCORPORATED |
| PASS OVER | PASS OVER | PASS OVER | PASS OVER | FILTER-TYPE | |
| SYSTEM | SYSTEM | SYSTEM | SYSTEM | HUMIDIFYING SYSTEM | |
| TUBES (INSPIRATORY CIRCUIT) | GENERIC (HEATED WIRED SYSTEM RECCOMENDED) | SPECIFIC (HEATED WIRED SYSTEM) | GENERIC | SPECIFIC (HEATED WIRED SYSTEM) | SPECIFIC TRIPLE LUMEN PATIENT CIRCUIT HEATED BY WATER RESERVOIR |
| INTERFASE | GENERIC | OPTIFLOW INTERFASE (SILICONE) | OPTIFLOW INTERFASE. (SILICONE) | SPECIFIC: (SLENDER SILICONE HEATED WIRED NASAL CANNULA) | Hi-VNI, Vapotherm: (SLENDER SILICONE CANNULA) |
| NASAL: 3 SIZES, REGARDLES THE FLOW SET | NASAL: 3 SIZES, REGARDLES THE FLOW SET | Standard-Plus: Flows up to 35L/m | TRACH MASK ADAPTER | ||
| Large: Flows up to 60L/m | |||||
| TRAQUEAL INTERFASE | TRAQUEAL INTERFASE | Tracheal: Flows up to 50L/m | |||
| ON-SCREEN MONITORING | SET FIO2 | MEASURED FIO2 | MEASURED FIO2 | MEASURED FIO2 | SET FIO2 |
| SET FLOW | SET FLOW | SET FLOW | SET FLOW | SET FLOW | |
| SET GAS TEMPERATURE | SET GAS TEMPERATURE | SET GAS TEMPERATURE | SET GAS TEMPERATURE | ||
| CONTINUOUS MONITORING OF PATIENT’S PARAMETERS: AIRWAY PRESSURE | CONTINUOUS MONITORING OF PATIENT’S PARAMETERS: NO | CONTINUOUS MONITORING OF PATIENT’’S PARAMETERS: NO | CONTINUOUS MONITORING OF PATIENT’S PARAMETERS: NO | CONTINUOUS MONITORING OF PATIENT’S PARAMETERS: NO | |
| SPO2 | |||||
| BPM | |||||
| RR | |||||
| ROX INDEX |
NHF: nasal high flow. FiO2: graction of inspired oxygen. O2: oxygen. SpO2: pulse oxygen saturation. BPM: beats per minute. RR: respiratory rate. ROX index: ratio of pulse oximetry/fraction of inspired oxygen to respiratory rate (SpO2/FiO2/RR).
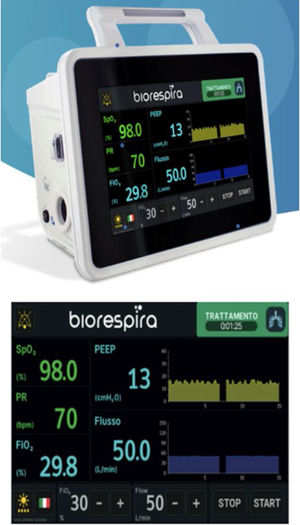
This innovative system is a “built-in high flow generator” with a turbine capable of generating high flows starting from the ambient air, mixed with medical oxygen that can be supplied via a hospital cylinder or medical gas plant. Despite being a turbine device, it guarantees a fixed FiO2 even when the flow changes. This control is based on an internal oxygen sensor and a valve that guarantees by opening and closing, the desired FiO2. It is the only turbine system that allows setting a precise FiO2, with a maximum achieved by 100%, independent of the variations of the flow setting. Originally, this device was invented for CPAP therapy, for this reason the flow range goes from 5 to 120L/m and at the same time, the device allows an accurate monitoring of the airway pressure. This wide range of flow enables the patient to perform a combined therapy with CPAP requirement (flows >60L/m) and NHF (flows <60L/m) during resting periods. Both the nasal cannula and the tubing and humidification system are generic, recommending the use of a pass over system with a heated wire circuit, to avoid condensation and heat loss from the inspired air. Other parameters that can be monitored, apart from the airway pressure, are respiratory rate, SpO2, heart rate and ROX index.
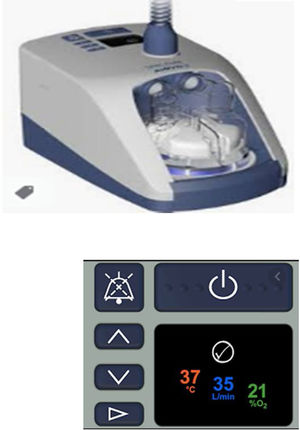
Airvo 2® (Fisher and Paykel)Fisher and Paykel defines this device as a “pass-over humidifier with integrated flow generator”. Its internal blower delivers high flow warmed and humidified gases to patients through a heated-wired circuit and a nasal cannula or a tracheotomy interface. Temperature can be set to three target dew-point settings in 31, 34 or 37°C and flows can be set between 10−60L/m. A flow meter up to 60L/m of supplementary oxygen can be connected to achieve FiO2 around 90%. It contains an oxygen analyser to determine the oxygen fraction delivered to the patient, affected by changes to the flow setting or supplemental oxygen setting. The nasal prongs and tubing between the nasal prong and heated-wired inspiratory circuit are both large bore, and the flow to the prongs is delivered from only one side.
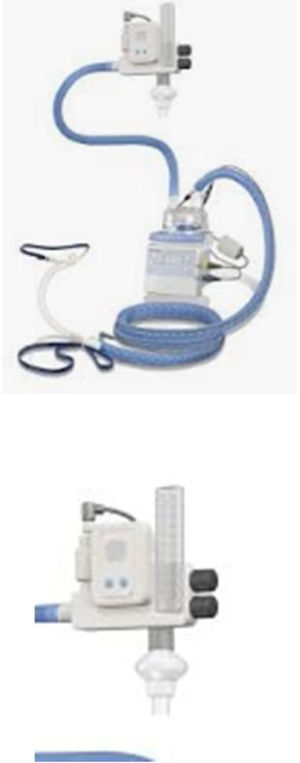
Optiflow® (Fisher and Pykel)This is a fixed-performance delivery device, also known as an “air-oxygen blender device”. With a blending system, separate pressurized air and O2 sources are input, and the gases are mixed with a precision valve (blender). This system allows a precise control over both FiO2 and total flow output. This blending system can provide flow rates from 30L/m to 60L/min and adjustable FiO2 from 21 to 100%. Humidification is achieved by a “pass over humidifier” connected to a heated wire circuit and a bore Nasal Cannula (Optiflow nasal cannula) or Trach Connection.
TNI softFlow 50® (Masimo)This device is a “built in flow generator” which generates the flow through an internal turbine. The applicator plug (circuit) is a silicone heated-wired circuit that enables a nasal application through a slender nasal cannula (also heated-wired and made of silicone) as well as a specific tracheal application. The TNI softFlow can be operated with two different humidifier types, but it always consists of a “pass-over system” chamber (Intersurgical®), with 1°C steps, within the range from 30 to 37°C. It can be used with the “Humidifier Clinic” for patients in clinics or in care facilities, or with the “Humidifier Homecare” for patients at home. When supplemental oxygen is required, an external, medically approved oxygen source can be connected to the TNI softFlow using the lateral oxygen inlet port. If no oxygen supply is needed, the oxygen inlet port must be kept sealed by the protective cap. The minimal operational flow is 10L/m, with 0,5L/m steps, to a maximum value of60L/m for a nasal application or 50L/m for tracheal application.
Precision Flow Vapotherm®The Precision Flow has an electronic gas mixer (proportional solenoid/flow valve) similar to that found in mechanical ventilators. For this reason, it requires a supply of high-pressure air and oxygen. This allows the device to use FiO2 between 21 and 100% regardless of the flow. It uses a different humidification system from the conventional pass-over humidifiers, which consists of a cartridge of steam transfer that allows no direct contact between the gas and water phases. Thus, gas and water are separated by a micro-porous membrane (0.005μm), which could represent a "protective barrier" for the gas flow to the patient. The circuit consists of a triple lumen supply tube: a central one, through which the gas flow circulates, surrounded by two external lumens through which hot water circulates to maintain the gas temperature (at the set temperature). This guarantees temperature uniformity (33°C–39°C range) throughout the circuit, reducing the possibility of condensation. The interface (Hi-VNI, Vapotherm) is a slender nasal cannula similar in appearance to a regular nasal oxygen cannula. Both the internal diameter and nasal prong bore are narrow, and this results in high flow out of the nasal prongs. The Precision Flow has a specially designed circuit to combine NHF with nitric oxide as well as helium-oxygen (Heliox) conditioned gas mixtures. This device has an internal battery. The battery takes 2h to fully charge for one hour of use.
In summary, NHF has recently gained attention as an alternative non-invasive respiratory support in several clinical scenarios. It is important to know the implementation technique of this treatment, as well as how to perform NHF with the different devices available in the market, because they each have different characteristics.
Conflicts of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.