Mepolizumab, a monoclonal antibody anti-IL-5, has been marketed in Portugal since 2017. We aimed to assess its effectiveness and safety in Portuguese severe eosinophilic asthmatic patients.
We conducted a single-center, observational, retrospective study, involving severe asthmatic patients under mepolizumab 100 mg subcutaneous, every 4 weeks for ≥12 months, from July 2017 to August 2020. Eligibility for treatment included a blood eosinophil count (BEC) ≥150/mm3 at baseline or ≥300/mm3 during the previous year. Demographic and clinical data were collected from the Portuguese Severe Asthma Registry (RAG) database. A written informed consent was obtained. BEC, Forced Expiratory Volume in one second (FEV1), exacerbation rate and oral corticosteroid (OCS) intake, as well as patient-reported outcomes (PROs) Asthma Control Test (ACT), Control of Allergic Rhinitis and Asthma Test (CARAT) and Mini Asthma Quality of Life Questionnaire (Mini-AQLQ) were accessed. Adverse events were documented. For statistical analyses (IBM-SPSS software, v25.0), t-independent and Mann-Whitney tests were used to compare parametric and non-parametric independent samples, respectively, while paired-t and Wilcoxon tests were employed to evaluate differences between intervals within the same variable, as appropriate. P-values <0.05 were considered statistically significant.
A total of 20 patients were enrolled in the study, mean age 54.0 ± 17.0 years [16–77 years], 13 were female (65%). Asthma diagnosis had been made, on average, 20 years before and severe asthma 10 years before. Mean BMI was 27.3 ± 5.4 Kg/m2 [19.5–38.3 Kg/m2], with six patients (30%) obese (BMI≥30 Kg/m2). Total serum IgE (median 274 kU/L, IQR 417.5 kU/L) was ≥100 kU/L in 16 patients (80%), with 10 patients (50%) showing positive skin prick testing (SPT). Rhinitis (N = 15, 75%), rhinosinusitis (N = 8, 40%) and nasal polyposis (NP) (N = 8, 40%) were the most common comorbidities (Table 1).
Baseline demographic and clinical characterization.
Data presented as n(%), mean±SD and median(IQR) as appropriate. BMI, body mass index; FEV1, forced expiratory volume in one second; GERD, gastroesophageal reflux disease; ICS+LABA, inhaled corticosteroid + long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; NSAID, non-steroidal anti-inflammatory drugs; OCS, oral corticosteroids.
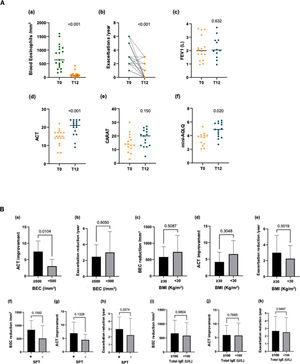
Regarding mepolizumab's efficacy (Fig. 1A), BEC significantly decreased from a mean of 753.2 ± 429/mm3 to 101.7 ± 102/mm3 (−86.5%, p < 0.001), as well as annual exacerbation rate (mean reduction 2.5/year, p < 0.001) and daily OCS intake with only two patients remaining under OCS after 12 months of mepolizumab (prednisolone 5 mg at alternate days, mean reduction 9.17 mg/day). There were no hospitalizations. Considering FEV1, only a slight improvement was observed (mean baseline of 2.1 ± 0.9 L to 2.2 ± 0.8 L, p > 0.05).
A: Changes in blood eosinophil count (a), exacerbation rate (b), FEV1 (Forced Expiratory Volume in one second) (c), ACT (Asthma Control Test) score (d), CARAT (Control of Allergic Rhinitis and Asthma Test) score (e) and mini-AQLQ (Mini Asthma Quality of Life Questionnaire) score (f) after 12 months of mepolizumab. B: Comparison of BEC (blood eosinophil count) reduction, ACT improvement and exacerbation rate reduction after 12 months of mepolizumab between different groups (baseline BEC≥500/mm3 vs <500/mm3, BMI (Body Mass Index) ≥30 Kg/m2 vs <30 Kg/m2, positive vs negative SPT (Skin Prick Testing), total serum IgE≥100 kU/L vs <100 kU/L).
Concerning PROs (Fig. 1A), statistically significant changes were observed in ACT (mean ∆-ACT 5.4 points, p < 0.001), with 16 patients (80%) presenting ≥20 points after 12 months of treatment, and mini-AQLQ (mean ∆-mini-AQLQ 1.3 points, p 0.02), while CARAT improvement did not reach statistical significance. Worth noting, 12-month mean upper airways score was lower compared with mean lower airways score (6/12 vs 12/18 points). NP was not monitored, although we did not find significant differences in ACT or exacerbations in these patients (p > 0.05).
A sub-analysis was attempted comparing changes in BEC, ACT and exacerbation rate after 12 months of treatment in patients with baseline BEC≥500/mm3 vs <500/mm3, BMI≥30 Kg/m2 vs <30 Kg/m2, positive vs negative SPT, total serum IgE≥100 kU/L vs <100 kU/L (Fig. 1B). There was a statistically significant difference (p < 0.05) between the groups of BEC≥500/mm3 vs <500/mm3 regarding changes in ACT.
Mepolizumab was well tolerated. Adverse events reported included myalgias in three patients, reverted with administration of magnesium, and persistent abdominal pain in one patient whose biological therapy was switched to another anti-IL-5.
Our study confirms mepolizumab's efficacy and safety, being the first study in a Portuguese cohort with severe eosinophilic asthma. These effects were similar in allergic and non-allergic patients, irrespective of total IgE serum concentrations. Patients with higher baseline BEC reported better symptom control, although annual exacerbation rate did not differ significantly.
We report a 76% improvement in annual exacerbation rate and a 92% reduction in daily OCS intake, which is higher than the reports of MENSA and SIRIUS clinical trials,1,2 but in line with other real-life studies.3–5 Similarly, a significant improvement in ACT score was documented, reaching not only statistical but clinical significance with ∆-ACT ≥3 points and 80% of patients adequately controlled (ACT≥20), as well as in quality of life. CARAT score did not reach statistical significance probably due to a less expressive improvement in nasal symptoms.
Despite real-life studies3,6 generally showing better results in FEV1 changes, ours failed to show significant improvement, probably due to the lack of reversibility in some patients. On the other hand, mepolizumab significantly reduced BEC in our cohort, suggesting a decrease in inflammation, with better results in patients with higher baseline values. However, larger real-life studies did not find statistically significant results.3,6,7
Regarding positivity in SPT or baseline total IgE, our results are in line with others previously reported,6 suggesting mepolizumab is effective in both allergic and non-allergic patients. Moreover, obese patients did not present significant differences in response to mepolizumab treatment compared to non-obese.
The limitations of our study include the reduced sample, limiting extrapolation of results, and its retrospective design, which could weaken our findings. However, it is the authors’ opinion that it has an important added value in providing the first real world evidence about the effect of mepolizumab in a cohort of Portuguese severe eosinophilic asthmatic patients.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.