Lymphomatoid granulomatosis (LYG) is a rare Epstein–Barr virus-driven lymphoproliferative disorder.1 It affects middle-aged adult males more often than females (M:F ratio≥2:1) and patients with underlying immunodeficiency are at increased risk.2
Pulmonary involvement occurs in over 90% of the patients and is usually present at initial diagnosis. Other common sites of involvement include the skin, peripheral and central nervous system, kidney and liver, whereas, the upper respiratory and gastrointestinal tract, lymph nodes, bone marrow and spleen may be also affected, but are relatively uncommon.3,4
Cough, chest pain and dyspnea are the main pulmonary symptoms and, hemoptysis usually indicates cavitation of the nodules. Constitutional symptoms including, fever, asthenia, night sweats and weight loss are common and few patients present with asymptomatic disease.4,5
Typical radiographic features are multiple bilateral lung nodules (80%), with predominant basal and peribronchovascular distribution, that can progress rapidly, coalesce and commonly cavitate. Additionally, LYG may present as pulmonary cystic lesion, pleural-based mass or prominent interstitial process.5
LYG histological evaluation is characterized by an angiocentric and angiodestructive infiltrating process, comprised of a mixture of small and large lymphocytes with variable cytologic atypia, histiocytes, and occasional plasma cells, lacking true granulomatous architecture.3,4 The infiltrate shows a predilection for vascular involvement and necrosis may be present3, and demonstration of EBV RNA genome or protein is a crucial point for correct diagnosis of LYG.
Despite relatively little evidence-based data, the WHO recommends that LG be graded in three different histologic types, according to the number of EBV-positive large B cells. This classification must be interpreted with caution, due to considerable diversity in sampling from the same individual and the possibility of different grades coexisting in the same sample. Atypical large B-lymphocytes proportion and to a lesser degree EVB-positive B-lymphocytes proportion help to classify the disease (grade 1–3) and give a prognostic value.6
We report a case of a 68-year-old male, former smoker with a medical history of cardiac heart failure, coronary heart disease, atrial fibrillation, type 2 diabetes, primary arterial hypertension and dyslipidemia.
The patient was admitted to the emergency department with recurrent syncopal events. Cranial computed tomography scan (CT scan) was unremarkable and CT pulmonary angiography revealed a pulmonary mass.
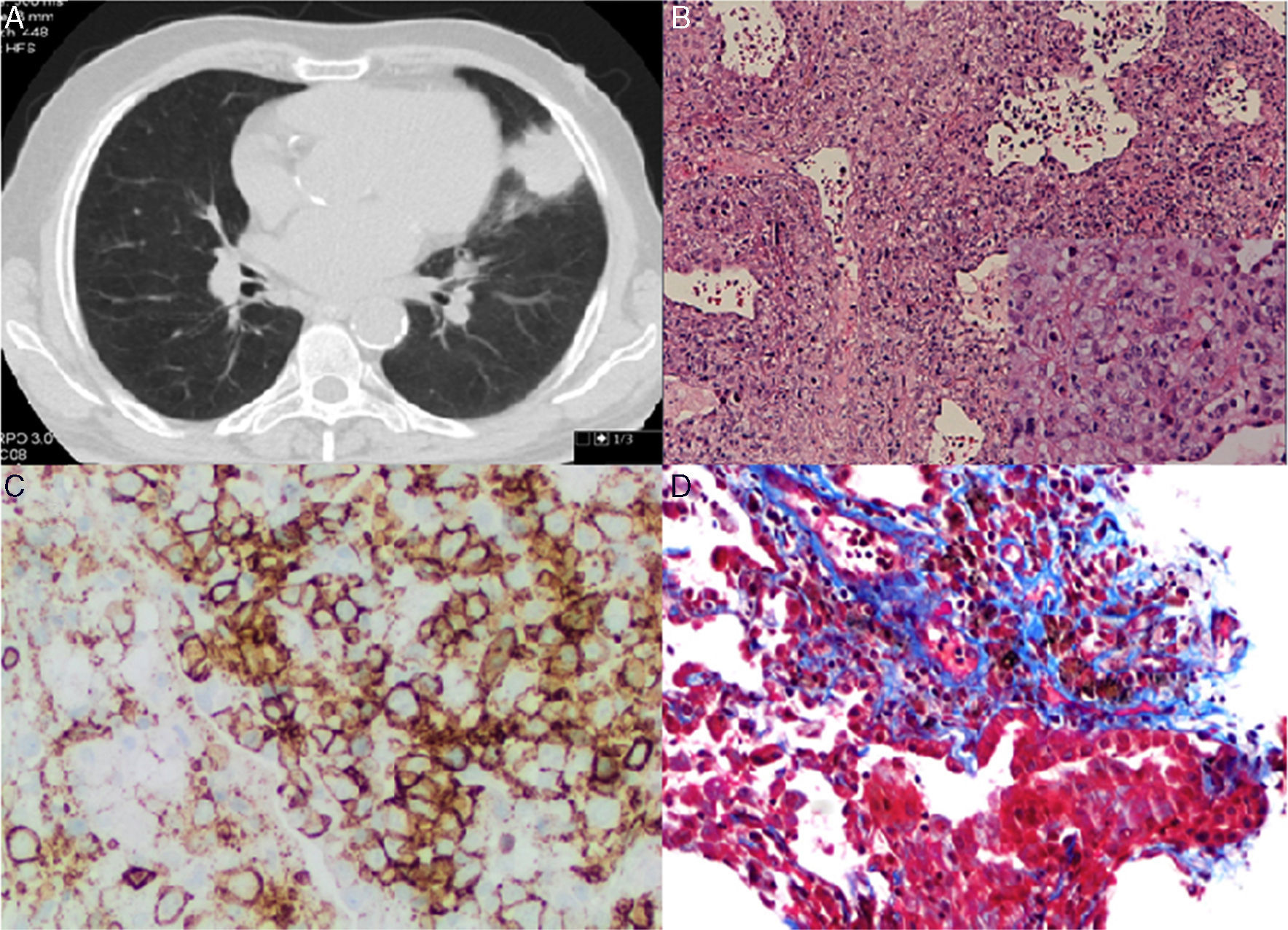
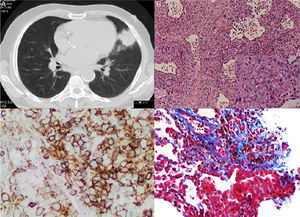
Chest and abdominal CT scan showed a mass of 4.3cm×3.7cm in the lingular segment, and three satellite nodules, the largest of which was 2cm in diameter. Several enlarged lymph nodes were identified (pre-vascular, paratracheal, subcarinal and left hilar) (Fig. 1A). Whole-body PET scan revealed high FDG uptake in the pulmonary mass, as well as, in two satellites nodules, in the left bronchohilar, mediastinal and right paratracheal lymph nodes and in the spleen.
(A) Chest CT scan showing a mass of 4.3cm×3.7cm in the lingular segment. (B) (HE 100×) – Polymorphic infiltrate in walls of blood vessels and alveoli, including large lymphocytes in aggregates (Inset: 400×). (C) Infiltrate with predominance of CD20+ B lymphocytes. (D) EBV-positive large atypical B cells.
CT-guided transthoracic core needle biopsy was performed and the histopathological examination revealed an interstitial polymorphic infiltrate of small T lymphocytes and large atypical B lymphocytes, with vascular wall permeation. The expression of EBV protein was identified in some of the large lymphocytes with imunnohistochemical staining (5–20cells per high-power field), also present in vascular walls (Fig. 1B–D). A diagnosis of lymphomatoid granulomatosis (grade 2) was established and the patient was referred to the Hematology department.
The clinical course of LYG is variable. Some patients follow a waxing and waning clinical course with rare spontaneous remissions without therapy. However, based on historical series, the majority of patients have progressive disease, with a median survival of two years. Predictors of poor prognosis are central nervous system involvement, grade 3, young age at diagnosis (<25 years), leukocytosis and hepatomegaly.7
No standard therapy has been established, and treatment depends on histologic findings as well as the extent and location of the disease. Grades 1 and 2 lesions may achieve durable responses to interferon-α 2b, whereas grade 3 is usually approached as diffuse large B-cell lymphoma.8
The authors present this case report based on its rarity and because it still remains relatively poorly recognized by clinicians and pathologists.5 Controversy still exists concerning diagnostic criteria, precise taxonomy, treatment and relationship to other lymphoproliferative diseases.4
Conflicts of interestThe authors have no conflicts of interest to declare.