Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia (DIPNECH) was first described in 1992.1 DIPNECH is a rare and poorly understood disorder that is believed to be underdiagnosed.2,3 It falls under the spectrum of neuroendocrine cell proliferations and it is characterised by marked and extensive hyperplasia of the neuroendocrine tissue of the bronchial and bronchiolar walls. It is considered a preinvasive lesion for lung carcinoid tumours by the World Health Organization.3 This proliferation of cells causes bronchiolar obstruction with subsequent constrictive bronchiolitis.
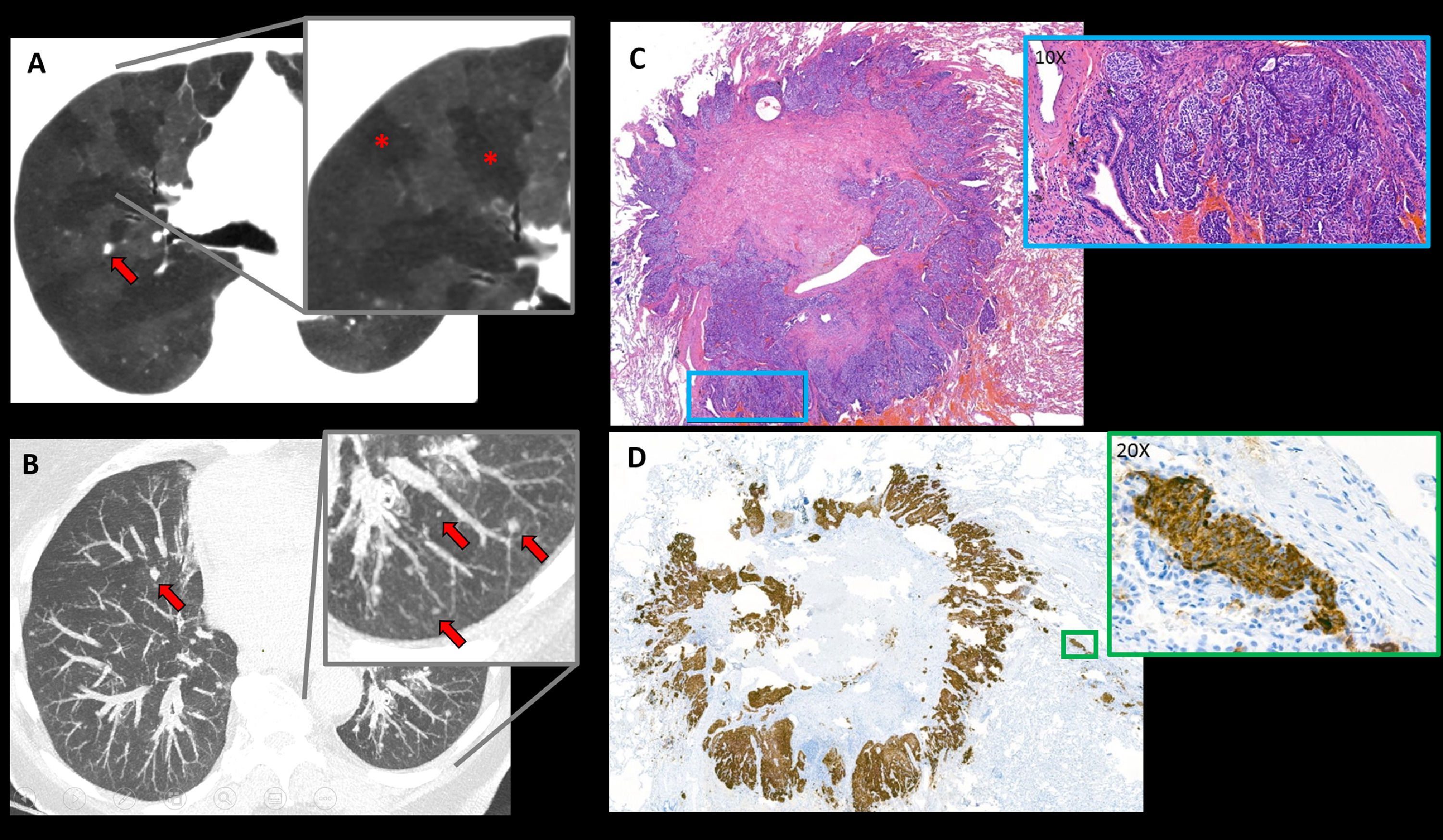
We present the case of a 75-year-old female who was referred to the pneumology department in the context of a history of more than one year of nocturnal non-productive cough associated with progressive dyspnoea. She reported no other symptoms. Stress echocardiography showed elevated systolic pulmonary artery pressure with exercise (47 mmHg). Functional respiratory tests showed no abnormalities. In order to rule out interstitial pathology, the study was completed with a chest High Resolution Computed Tomography (HRCT) that showed multifocal bilateral pulmonary micronodules, bronchiectasis with bronchial wall thickening and extensive mosaic attenuation areas due to air trapping (Fig. 1A,B). DIPNECH diagnosis was proposed. The patient underwent video-assisted wedge lung resection thoracic surgery. The histologic description showed a clustered intramucosal proliferation of spindle-shaped cells with salt and pepper chromatin and positivity to neuroendocrine markers (Fig. 1C,D).
Axial chest HRCT images: (A) MinIP image showing a mosaic attenuation pattern suggestive of air trapping (red asterisks in detail image). (B) MIP image showing countless bilateral millimetric pulmonary nodules (red arrows). (C) Haematoxylin-eosin stain of lung wedge resection fragment: a subpleural carcinoid tumour is seen, in the magnified image x10 high power fields (HPF) cells with neuroendocrine features can be seen: spindle-shaped, without mitotic figures or necrosis (blue box). (D) Chromogranin stain (neuroendocrine marker) shows diffuse positivity in nests of neuroendocrine cells (x20 HPF) (green box).
DIPNECH has been typically described in middle-aged, non-smoking women.4,5 Although it is most often an incidental finding, there is the possibility of its presentation as an insidious clinical picture of dyspnoea and dry cough. On HRCT, DIPNECH manifests as multiple small bilateral pulmonary nodules that represent neuroendocrine cell aggregates that can narrow the lumen of the distal airway with subsequent thickening of the bronchial walls and, therefore, bronchiectasis, mucus plugs and air trapping develop. Histologic analysis gives the definitive DIPNECH diagnosis.4 Aggregates of round, oval or spindle-shaped neuroendocrine cells with salt-and-pepper chromatin and immunoreactivity to chromogranin and synaptophysin are observed. The rarity of this condition poses some clinical challenge and DIPNECH treatment must be individualised. It can have a variable prognosis, but most studies show a good clinical outcome with follow-up and observation.3,4 There is no evidence to support treatment with chemotherapy.3 Surgical resection is possible if any area progresses to carcinoid tumours during follow-up.
The authors have not received any funding.
The authors contributed in equal proportion to the realisation of the article.