Nontuberculous mycobacteria (NTM) are usually classified based on the time to colony formation in subculture: rapidly-growing mycobacteria (RGM) (<7 days), and slowly-growing mycobacteria (SGM) (≥7 days).1,2
Clinical patterns of disease may vary – lung infection in patients with structural disease is mostly caused by SGM, although RGM may display a similar clinical and radiological presentation. The pattern of disseminated disease is also somewhat different: RGM usually spread from a contaminated source with direct inoculation, most commonly manifesting as skin and soft tissue infection. Conversely, SGM typically affect severely immunocompromised patients, mostly affecting deep lymph nodes, liver and spleen.
Our main goal was to identify relevant features associated with an increased risk of either RGM or SGM infection. Drug susceptibility,3 treatment recommendations, and outcome differ between the two groups of NTM and specific pathogens.1 Identifying differential risk factors may allow a more timely initiation of empirical treatment while awaiting the mandatory identification of species and drug susceptibility testing (DST).
A retrospective analysis of all reported cases of NTM infection between January 1st 2003 and March 31st 2016 was conducted. In Portugal, many NTM infections are managed in the same outpatient centers as tuberculosis, mostly because care and antimicrobial drugs are provided free of charge. Those who fulfill the ATS/IDSA 2007 criteria4 and start treatment are then notified and reported with relative detail, providing reliable estimates of the number, clinical picture and comorbidities of cases of NTM infection.
Demographic (gender, age, occupation and country of origin), and clinical data (past history of TB, comorbidities or other risk factors, and site of infection) were collected from the Public Health authorities’ records. Microbiological data (species, smear, culture and nucleic acid amplification testing [NAAT] results) were collected from the national reference laboratory for mycobacterial infections at Doutor Ricardo Jorge National Institute of Health. Approval by institutional Ethics Committee was not required.
Associations between SGM or RGM infection and categorical variables of interest were evaluated by chi-square (or Fisher's) test, while associations with quantitative variables were studied using Mann–Whitney U test. Multivariate analysis comprised logistic regression models. The statistical analyses were performed with the R language and software environment for statistical computation, version 2.3.3. The significance level was set at 0.05.
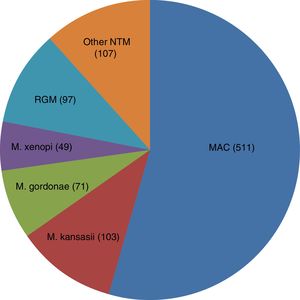
From a total of 953 isolates, 938 clinically relevant cases were analyzed: 734 (73.8%) due to SGM and 97 (10.7%) to RGM. The most frequent SGM was Mycobacterium avium complex (MAC), and M. chelonae the most frequent RGM (Fig. 1). In 107 (11.4%) cases, the causative species was not determined (excluded from further analysis).
The distribution of NTM disease based on the site of infection and culture results is shown in Table 1: 328 (34.9%) patients were smear-positive, and 692 (73.8%) were culture positive. Culture growth in respiratory samples was significantly better in RGM than in SGM (83.9% vs 63.5%), and respiratory samples were the most frequent culture growth product of RGM (OR=2.591, CI95% 1.43–4.76). SGM were more frequent in HIV-infected patients (OR=3.300, CI95% 1.33–8.33), whereas RGM were more frequent in those with a precarious social status, defined by either past or present homelessness or incarceration (OR=4.672, CI95% 1.82–11.99) (Table 1).
Risk factors for nontuberculous mycobacterial infections.
| Demographic variable | RGM infection (%) | SGM infection (%) | Total (%) | p-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 62 (63.9) | 434 (59.1) | 496 (59.7) | 0.367 |
| Female | 35 (36.1) | 300 (40.9) | 335 (40.3) | |
| Age | ||||
| Mean (±SD) | 64 (±17.1) | 58 (±18.6) | 56 (±18.2) | 0.121 |
| Occupation | ||||
| Active | 27 (36.0) | 259 (45.7) | 286 (44.5) | 0.092 |
| Retired or unemployed | 48 (64.0) | 308 (54.3) | 356 (55.5) | |
| Origin | ||||
| Portuguese | 91 (93.8) | 665 (90.6) | 756 (91.0) | 0.395 |
| African | 4 (4.1) | 46 (6.3) | 50 (6.0) | |
| South American | 1 (0.1) | 11 (1.5) | 12 (1.4) | |
| European | 1 (0.1) | 10 (1.44) | 11 (1.3) | |
| Other | 0 (0.0) | 2 (0.2) | 2 (0.3) | |
| Clinical | ||||
| Chronic lung disease | 13 (13.4) | 107 (14.6) | 120 (14.4) | 0.757 |
| Chronic kidney disease | 2 (2.1) | 5 (0.7) | 7 (0.8) | 0.211 |
| Chronic liver disease | 0 (0.0) | 25 (3.4) | 25 (3.0) | 0.175 |
| HIV infection | 9 (9.3) | 167 (22.8) | 176 (21.2) | 0.003 |
| Cancer | 9 (9.3) | 38 (5.2) | 47 (5.7) | 0.105 |
| Diabetes mellitus | 7 (7.2) | 32 (3.4) | 39 (4.7) | 0.216 |
| Rheumatic disorder | 1 (1.0) | 13 (1.8) | 14 (1.7) | 0.861 |
| Past history of tuberculosis | 15 (15.5) | 140 (19.1) | 155 (18.7) | 0.392 |
| Behavioral | ||||
| Precarious social status | 9 (9.6) | 26 (3.7) | 35 (4.4) | 0.013 |
| Intravenous drug use | 4 (4.1) | 77 (10.5) | 81 (10.4) | 0.256 |
| Alcohol abuse | 9 (9.9) | 81 (12.1) | 90 (11.8) | 0.549 |
| Site of infection | ||||
| Pulmonary | 83 (85.6) | 606 (82.6) | 689 (94.3) | 0.839 |
| Pulmonary andExtrapulmonary | 1 (1.0) | 36 (4.9) | 36 (4.3) | |
| Extrapulmonary | 13 (13.4) | 93 (12.7) | 106 (12.8) | |
| Pleural | 1 (1.0) | 23 (3.1) | 24 (2.9) | |
| Lymphatic | 2 (2.1) | 20 (2.7) | 22 (2.6) | |
| Intraabdominal | 1 (1.0) | 5 (0.7) | 6 (0.7) | |
| Genitourinary | 2 (2.1) | 10 (1.4) | 12 (1.4) | |
| Bone and joint | 1 (1.0) | 12 (1.6) | 13 (1.6) | |
| Disseminated | 2 (2.1) | 38 (5.2) | 40 (4.8) | |
| Other | 5 (5.1) | 20 (2.7) | 25 (3.0) | |
| Positive culture | 87 (89.7) | 635 (86.5) | 722 (86.9) | <0.001 |
| Respiratory sample | 73 (83.9) | 403 (63.5) | 476 (65.9) | |
| Other sample | 14 (16.1) | 232 (36.5) | 246 (34.1) | |
There were no statistically significant differences between SGM and RGM infected patients regarding any of the studied demographic variables (Table 1).
MAC and M. kansasiiinfections are AIDS-defining events, making the stronger association between HIV infection and SGM unsurprising. HIV infection was the most commonly reported comorbidity in our study, which might partly explain the relatively lower rate of RGM infection in our sample.
Precarious social living conditions are known risk factors for other contagious diseases, particularly airborne infections such as measles and TB.5,6 Recent findings pointing to human-to-human transmission of M. abscessus have raised the concern regarding RGM infections in specific populations.7 Studies suggest that RGM possess virulence factors that enhance their adherence to the ciliary epithelium, and molecular pathways that promote growth when in respiratory cells and mucus environment.8
One limitation of our study is its retrospective nature and missing data (it was not possible to identify specific environmental, occupational or nosocomial exposure, and more detailed clinical data; not only that, more than 10% of NTM isolates were not specified). Despite these limitations we describe a nationwide sample with somewhat detailed demographic, laboratory and clinical data.
Conflicts of interestThe authors have no conflicts of interest to declare.
Rita Gaio was partially supported by CMUP (UID/MAT/00144/2013), which is funded by FCT (Portugal) with national (MEC) and European structural funds (FEDER), under the partnership agreement PT2020.