The war against Covid-19 is far from won. This narrative review attempts to describe some problems with the management of Covid-19 induced acute respiratory failure (ARF) by pulmonologists.
MethodsWe searched the following databases: MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and reviewed the references of retrieved articles for additional studies. The search was limited to the terms: Covid-19 AND: acute respiratory distress syndrome (ARDS), SARS, MERS, non invasive ventilation (NIV), high flow nasal cannula (HFNC), pronation (PP), health care workers (HCW).
ResultsProtection of Health care workers should be paramount, so full Personal Protective Equipment and Negative pressure rooms are warranted. HFNC alone or with PP could be offered for mild cases (PaO2/FiO2 between 200–300); NIV alone or with PP may work in moderate cases (PaO2/FiO2 between 100–200). Rotation and coupled (HFNC/NIV) strategy can be beneficial. A window of opportunity of 1–2h is advised. If PaO2/FIO2 significantly increases, Respiratory Rate decreases with a relatively low Exhaled Tidal Volume, the non-invasive strategy could be working and intubation delayed.
ConclusionAlthough there is a role for non-invasive respiratory therapies in the context of COVID-19 ARF, more research is still needed to define the balance of benefits and risks to patients and HCW. Indirectly, non invasive respiratory therapies may be of particular benefit in reducing the risks to healthcare workers by obviating the need for intubation, a potentially highly infectious procedure.
“You are coming to fight against me with a sword, a spear and a javelin. But I’m coming against you in the name of the Lord who rules over all. He is the God of the armies of Israel. He's the one you have dared to fight against” 1 Samuel 17
While the world is racing to contain the spread of COVID-19 and updated/real time medical information has reached high ranked journals faster than ever, there are still a lot of questions unanswered. The huge efforts made by some countries have allowed us to gain critical time for better preparation and increase our awareness.
In fact some reflections could help pulmonologists tackle the current pandemic. Reports from China suggest that 81% of COVID-19 are mild, 14% are severe and that 5% require intensive care.1 Mortality rate in the series published from China, Italy and US,2–16 ranges from 1.4% in hospitalized6 to 61.5% in critically ill patients.10
In this frame the role of pulmonologists is increasing. This narrative review tries to describe some problems with the management of Covid-19 induced acute respiratory failure (ARF) by pulmonologists, remaining aware that the overflow of new information may make all reports rapidly obsolete.
Data sources and search strategiesWe searched the following databases: MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, from 2010 to April, 15th 2020, with no language restriction. We also reviewed the references of retrieved articles for additional studies. The search was performed using the terms: Covid-19 AND: acute respiratory distress syndrome (ARDS), non invasive ventilation (NIV), high flow nasal cannula (HFNC), pronation, chest physiotherapy, health care workers, severe acute respiratory syndrome (SARS), influenza A H1N1, Middle East respiratory syndrome (MERS).
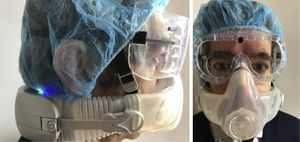
Protection of health care workersOne of the most relevant, and unfortunately neglected (at least at the beginning) problems is the protection of professionals involved in high-risk interventions such as nebulizer therapy, HFNC, oxygen therapy, NIV, patient pronation, chest physiotherapy. According to the available reports, 3.8% of Chinese health care workers were infected,1 63% of cases occurring in Wuhan; In Italy the figures are worse with 14% of cases.17 How can we reduce the impact on these professionals? The fundamental defense is to wear effective protective personal equipment such as N95 masks, gowns hair covers, gloves, eye and face shields.18 The use of more efficient respirators (Powered Air Purifying Respirators) for high risk aerosol generating procedures (like respiratory therapies) has been proposed19 (Fig. 1).
Non invasive ventilation and high flow nasal cannulaThe ERS/ATS clinical practice guidelines recommend (not firmly) NIV as a preventive strategy for avoiding intubation in hypoxemic ARF20 only when performed by experienced teams in highly selected cooperative patients with community-acquired pneumonia or early ARDS without any associated major organ dysfunction.
In patients with de novo ARF under NIV, large expiratory tidal volumes (VTE) may be generated in assisted pressure controlled modes by the ventilator pressure and the one generated by the respiratory muscles. Therefore reliable monitoring of VTE and unintentional leaks would be of outmost importance. When using an ICU ventilator driven by high pressures in the double limb configuration, leaks are computed as the difference between inspired tidal volume and VTE. As a consequence, the amount of tidal volume that the patient gets is usually quantified as VTE.21 In the majority of patients with “de novo” moderate-to-severe hypoxemia, a targeted VTE of 6–8mL/kg was impossible to achieve by NIV with humidified masks and ICU ventilators.22 A higher VTE was independently associated with NIV failure. In the subgroup of patients with an arterial oxygen tension to inspiratory oxygen fraction (PaO2/FiO2) ratio up to 200, a mean VTE higher than 9.5mL/kg over the first four cumulative hours of NIV accurately predicted NIV failure.22 A higher VTE was independently associated with NIV failure. In the subgroup of patients with an arterial oxygen tension to inspiratory oxygen fraction (PaO2/FiO2) ratio up to 200, a mean VTE higher than 9.5mL/kg over the first four cumulative hours of NIV accurately predicted NIV failure.
In a recent randomized controlled trial, Patel et al.,23 found that NIV delivered via helmet reduced intubation rates in patients with ARDS more significantly, compared to NIV delivered via facial mask (from 61% to 18%, respectively). As the helmet seems a more effective and tolerable interface in this setting, it would make sense to evaluate how it stands compared to HFNC.24 Indeed, very recently, a physiological randomized cross-over study,25 concluded that in patients with PaO2/FiO2 <200, high-PEEP helmet NIV could be preferred over HFNC to optimize oxygenation and mitigate the inspiratory effort, especially in most severely hypoxemic patients and in those exhibiting intense inspiratory effort during HFNC. Caution is needed in patients with low inspiratory effort during HFNC, because they can experience increases in dynamic transpulmonary driving pressure while on NIV with the helmet.
A recent systematic review26 shows that compared to conventional oxygen therapy HFNC decreases risk of requiring intubation without impacting mortality. The authors pointed out that flow rates were variable between the studies and also, duration of treatment was not analyzed. A physiologic randomized controlled study27 showed that the higher (60Lmin−1) the flow, the better the physiologic response.
PronationAdding prone positioning to HFNC, Riera et al, Riera et al.,28 demonstrated, in healthy subjects, that it leads to a more homogeneous distribution of end-expiratory lung impedance possibly translating into better oxygenation.
In awake, non-intubated, spontaneously breathing patients with hypoxemic ARF (majorly immunocompromised) Scaravilli et al.,29 showed a significant improvement in PaO2/FiO2 with prone positioning. More recently, early prone positioning added to HFNC or NIV avoided the need for intubation in up to half of the patients with moderate to severe ARDS including those with viral pneumonia.30 No health care professional was infected during this study carried out in isolation negative pressure rooms. Other authors report similar results31 and a randomized controlled trial is ongoing.32
Combination of both NIV and HFNCFrat JP et al.,33 in patients with PaO2/FiO2 <300, studied the effect of sequential application of sessions of HFNC and NIV. Intubation was required in 36% of patients, including individuals with ARDS. Authors concluded that due to the good tolerance and efficacy on oxygenation, HFNC could be a good option to be used between NIV sessions to pursue a coupled non invasive strategy of ventilation without a marked impairment of oxygenation.
NIV and HFNC in a pandemic context. Lessons learned from SARS, H1N1 and MERSIn 2003, NIV was tried in Hong Kong in patients with ARF (initial mean PaO2/FiO2 137) secondary to SARS outbreak. The treatment was carried out in isolation/negative pressure rooms, with a Bi-Level ventilator in spontaneous/timed mode, oro-nasal masks and single circuit with the filter before the expiratory valve. Endotracheal intubation was avoided in 70% of cases and one month after the outbreak no health care worker (wearing fully fledged protective personal equipment, including Powered Air Purifying Respirators) was infected.34 Non invasive ventilation was also used in patients with ARF due to influenza A H1N1 infection, with success rates ranging between 15% and 25%.35,36
More recently, during the MERS outbreak Alraddadi et al.,37 showed that NIV as first line intervention, although modestly effective was not associated with increased 90 day mortality. No data was available on potential risks of transmission to health care professionals. Considering the usage of HFNC in the pandemic context, Rello et al.,38 reported cases of ARF due to the pneumonia 2009 influenza A/H1N1v, with a success rate of 39%, and no secondary infections in health care workers (even without negative pressure rooms). Moreover reports from China in MERS show the effectiveness of HFNC with apparently no transmission reported to the professionals.39,40
Risk of different interventionsBefore choosing the best respiratory support for patients with ARF, we need to understand the risks of different interventions. A systematic review concluded that the most consistent association with increased risk of SARS transmission to professionals was tracheal intubation; mask ventilation was also positively associated (only 2 studies) but data were not considered sufficiently robust to establish firm conclusions.41
As shown in Table 1, bench studies showed that dispersion of exhaled air is different depending on the respiratory therapies and interfaces (nasal cannula, oro-nasal mask or helmet).42–46 Oxygen delivered at 6L/min in a mild lung injury model had maximum dispersion distance of 0.22m from the mask.42 When the experimental setting analyzed the performance continuous airway positive pressure (CPAP) with the Quattro Air mask (ResMed®) there was no significant leakage when pressures up to 20cmH2O were applied. In fact, exhaled air dispersed evenly via the vent holes located circularly around the elbow connection point in all directions at very low normalized smoke concentration <20%.43 With Nuance Pro Gel (Philips-Respironics®) and Swift FX (ResMed®) nasal pillows there was a significant increase in exhaled air dispersion distance for both nasal pillows with increasing CPAP (0.19–0.21m).43
Exhaled air dispersion according with modalities and interfaces.43–46
| Interfaces and pressures (in cmH20) | Maximum exhaled air distance (in meters) |
|---|---|
| ResMed Ultra Mirage mask IPAP/EPAP cmH2O | |
| 10/4 | 0.40 |
| 14/4 | 0.42 |
| 18/4 | 0.45 |
| ResMed Quattro Air mask (with anti-asphixia valve closed) | |
| CPAP 10–20 cmH20 | Negligible |
| Respironics Total Face IPAP/EPAP cmH20 | |
| 10/5 | 0.61 |
| 18/5 | 0.81 |
| Helmet StarMed CaStar R IPAP/EPAP cmH20 | |
| IPAP from 12 to 20/EPAP 5 | Negligible |
Abbreviations: CPAP, continuous positive airway pressure; IPAP, inspiratory positive airway pressure; EPAP, expiratory positive airway pressure.
Regarding the performance of Bi-Level modes, substantial exposure to exhaled air occurs within a one meter region, from patients receiving NIV through oro-nasal masks with integrated expiratory valves or oro-nasal masks attached to an expiratory valve, with more diffuse leakage from the latter, especially at higher IPAP.44 A different oronasal mask with integrated expiratory valve located on the upper part of the mask (Ultra Mirage Medium; ResMed®) led to substantial exposure to exhaled air occurring within a 0.5-m radius of patients receiving NIV.45 With the Philips-Respironics® total face mask early model, exhaled air jet through the integrated exhalation port could reach a distance of 0.92m when NIV was applied using single circuit.46
With the StarMed CaStar R Helmet, leakage of exhaled air was negligible when NIV was applied with a double limb circuit, filters and a good seal at the neck-helmet interface, whereas leakage at the neck interface could reach a maximum radial distance of 0.27m through another helmet (Sea-Long model) without a tight seal in the interface.46
Using a real human model (with healthy controls, subjects with coryzal symptoms and patients with infective COPD exacerbations) Simonds et al.,47 showed that NIV using a vented mask produced droplets in the large size range (>10μm) compared with the baseline droplet counts (without any intervention). This increase in large droplets was not seen using the NIV circuit modification (with non-vented mask and exhalation filter). Oxygen therapy did not increase droplet count in any size range.
From these studies, we might conclude that NIV through the helmet with double limb circuit and a good seal at the neck-helmet interface would be a safe option for managing infectious patients with hypoxemic ARF. As alternative, the Quattro Air mask (ResMed®), or a non-vented oro-nasal mask with a bacteriologic filter at the circuit's expiratory valve could be the more efficient alternatives.
High flow nasal cannulaStudies coming from the above mentioned laboratory,43 showed that with HFNC (model Airvo 2; Fisher & Paykel®) exhaled air mean distances increased from 65 to 172mm when flow was increased from 10 to 60Lmin−1, a shorter distance than that from application of CPAP through the commonly used nasal pillows. Moreover air leakage to 620mm occurred laterally when HFNC and the interface tube became loose.43 In another experiment with a manikin and no negative pressure room, 10min of HFNC at 60L/min of flow, caused no dispersal of water yeast in areas >60cm away from the face. Manual repositioning of cannula slightly increased dispersal.48
In critically ill patients with Gram negative Pneumonia, in single occupancy negative pressure rooms, there was no difference in bacteria count between HFNC and Venturi Mask at 0.4m and 1.5m plates.49 In an experiment with healthy volunteers to simulate a patient coughing while using HFNC to assess the maximum distance of droplet dispersion, Loh et al.,50 showed that while wearing a well-fitting nasal cannula at 60Lmin−1 flow, cough generated droplets spread up to a distance of 4.50m. To circumvent some of the risks of HFNC, some authors propose that the patient wear a surgical facemask on top of the nasal cannulas.51 A recent simulation of HFNC along with a surgical facemask in place over the cannula, confirms that, at 40Lmin−1, the surgical mask captured 83.2% of particles, at the expense of a moderate reduction in CO2 clearance.52 This may require increasing flow rate of HFNC if the patient is displaying increased work of breathing.
NIV and HFNC usage in the current COVID-19 pandemicAnalyzing current trends in NIV and HFNC usage in all published series in major journals shows the following,2,15,16 mean NIV usage in hospitalized patients in China was 20.1% (from 4.9 to 56%; higher in series including only critically ill,10 moderate to severe14 and in one series of Pneumonia cases11); in Italy 11%15; and in USA from 0 to 19%.12,13 Mean HFNC usage in China was 22.8% (raging from 0 to 63.5% and higher in series including only critically ill10 and moderate to severe14), in Italy15 0%, and in the USA from 4.8% to 42% (higher in Critically ill in Seattle13 than in Washington-12). In a real world study in two Chongqing hospitals in China53,52 of the patients experiencing severe ARF, 63% of patients were treated with HFNC as first-line therapy, 33% were treated with NIV and 4% were treated with invasive ventilation. Of the HFNC patients, 41% experienced failure, failure rate being 0 in patients with PaO2/FiO2 >200 and 63% in those with PaO2/FiO2 ≤200.
One important issue about HFNC is that the amount of condensation in the circuit increases when the ambient temperature decreases. At present, the condensed water can become an important source of infection for COVID-19 so, avoidance or reduction in condensation may be very important when HFNC is used. Bacterial contamination of the inner surface of circuits after termination of HFNC has been shown in 16.1% of cases, mainly occurring at the interface end. This figure is as high as for anesthetic breathing circuits, but can be decreased by circuits fitted with heating wires, which greatly reduce condensation.54
Although the evidence from the recent series is lacking (with no single mention of transmission to professionals through these techniques), there are authors that do not recommend NIV or HFNC until the patients is cleared of COVID-19.55 However some other experiments from China suggest that early intervention with HFNC and NIV associated or not with prone positioning can lead to lower mortality, less than 1% of cases needing intubation (versus 2.3% of National average).53
Potential strategies and perspectivesOptions differ between Scientific Societies56–63 (Table 2), countries and different environmental factors.64 In fact, lack of facilities, ICU beds, experienced personnel,65 or equipment66 may have a role in selecting these therapies and ultimately will have an impact in clinical outcomes.
Respiratory non-invasive therapies for COVID-19: recommendations from scientific societies.
| Scientific society (country), ref nr | Non-invasive respiratory therapy first option |
|---|---|
| SEPAR (Spain)56 | HFNC |
| AIPO (Italy)57 | Helmet CPAP |
| ESICM/SCCM (EU/US)58 | HFNC |
| SPP (Portugal)51 | HFNC or CPAP |
| NHS (UK)59 | CPAP |
| WHO60 | HFNC or NIV |
| CTS (China)61 | HFNC |
| ANZICS (Australia/New Zealand)62 | HFNC |
| Multiple Societies (Germany)63 | Helmet NIV |
Abbreviations: SEPAR-Sociedad Española de Patologia Respiratória; AIPO-Associazone Italiana Pneumologi Ospedalieri; ESICM-European Society of Intensive Care Medicine; SCCM-Society of Critical Care Medicine SPP-Sociedade Portuguesa de Pneumologia; CTS-Chinese Thoracic Society, ANZICS-Australian and New Zealand Intensive Care Society.
Effectiveness of NIV or HFNC as first line needs a deeper evaluation, and also whether the early use of invasive ventilation can really improve prognosis. Two different phenotypes of patients have been hypothesized67: more than 50% of COVID-19 pneumonia with Berlin criteria of ARDS have normal lung compliance, with “silent” hypoxemia (the so called Type L phenotype); these patients when non dyspnoeic should just receive supplemental oxygen; if dyspnoeic, should be offered HFNC, CPAP or NIV. If the patient shows significant increase in work of breathing, we should proceed to intubation and invasive mechanical ventilation.67 A clear analysis of NIV/HFNC time before invasive mechanical ventilation is most important. Indeed some authors that NIV has a role in self inflicted lung injury (SILI) and the risk of impacting in a change in ARDS phenotype (Paolo Pelosi, personal communication, Webinar ESICM). The extended effect of heated and humidified oxygen in HFNC to avoid mucosal injury, improve secretion clearance, reduce transpulmonary driving pressure, should also be looked at in this setting.68 Its role in preventing injury and accelerate recovery if initiated early in the clinical course should be also analyzed.
Non invasive ventilation and HFNC can be reserved for patients with mild ARDS, with close monitoring, airborne precautions, and preferably in single rooms. In patients with suspected or diagnosed COVID-19 requiring NIV, helmets may be the best solution for CPAP or NIV, because of minimal or no dispersion from leaks, easy to filter/scavenge exhausted gas. Due to the scarcity of this interface it is probable that traditional oro-nasal masks will be the most commonly used. In this case suboptimal NIV set-up, with interface with inappropriate seals and improper circuitry will not be tolerable. If NIV is the option, try “protective-NIV” with lower tidal volumes between 6 and 8mL/kg.69
This simple description of some problems of this narrative review elicits the need for innovative strategies70 in addition to medical therapy and vaccination campaigns.
ConclusionAll respiratory therapies represent a risk of aerosol generating procedures during the care of patients with COVID-19. Personal Protective Equipment and Environmental Control/Engineering should be the initial concern and consideration when managing patients with COVID-19. Given the current circumstances it is not likely that there will be randomized controlled trials to confirm which non invasive respiratory support is better to reduce the need for intubation in the context of COVID-19 pandemic. Manufacturers should be urged to create safer interfaces, viral proof circuitry and “new generation” non invasive ventilators with integration of different therapies, specific monitoring and necessary safety features. It is our impression, that this will be a marathon not a sprint, and like David we must beat Goliath.
Conflicts of interestThe authors have no conflicts of interest to declare.