Worldwide, atrial fibrillation (AF) affects 1-2% of the population, and left atrium (LA) transcatheter linear radiofrequency (RF) ablation is an established therapeutic option in this subset of patients.1,2 The rationale behind this technique is consequent to the fact that premature atrial ectopic beats in AF originate predominantly from the atrial myocardial sleeves and extend into the pulmonary veins. RF energy at the junctions between the pulmonary venous ostia and the LA can electrically isolate the arrhythmogenic sources of ectopias, eliminating the AF trigger.3 However, transcatheter RF ablation has been associated with rare but life-threatening complications, such as pulmonary vein stenosis (PVS).4,5 The clinical profile and computer tomography (CT) features of this complication have been previously described,6 but data on radiological-pathological correlations are still limited. Here we present two cases of PSV after transcatheter RF ablation in which histopathological features were obtained. Patients signed a consent form for publishing their clinical data anonymously.
Clinical data. Patient N 1. A 48-year-old non-smoker female nurse, presented with a history of waxing and waning left lower lobe consolidations, recurrent fever, moderate dyspnea on exertion and left chest pain. She had been treated with RF ablation 14 months earlier for a paroxysmal AF. Pulmonary function tests (PFT) showed only a slight decrease of DLCO (62% of predicted). Laboratory tests revealed mild leukopenia, moderate anemia with normal CRP.
Patient N 2. A 50-year-old non-smoker tradesman sought medical consultation for hemoptysis. He had been submitted to RF ablation 10 months earlier for an uncontrolled paroxysmal AF. PFT were unremarkable and laboratory tests documented only a mild anemia.
Both patients underwent a CT scan and a transbronchial cryobiopsy.7 In case N 2 a pleural biopsy was also carried out.
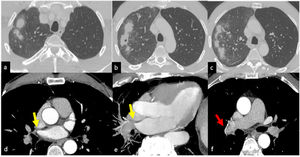
Imaging features. In both cases CT scan showed multiple parenchymal ill-defined rounded opacities in the subpleural regions and smooth peripheral thickening of the interlobular septa. The mediastinal window of the CT scan showed a stenotic aspect of pulmonary veins. Furthermore, in case N 2 a significant pleural thickening associated with loculated effusion was documented (Fig. 1).
Ill-defined, rounded and partially confluent consolidations with halo sign are present in the right upper lobe. Mild alveolar ground glass attenuation is present in the surrounding parenchyma (a-c). Tiny cavitation is visible in one of the consolidated lesions (b). Axial images with mediastinal window and MIP reconstructions show right upper pulmonary vein stenosis (d, e - yellow arrows) associated with hypertrophy of bronchial arteries (f - red arrow).
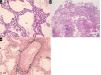
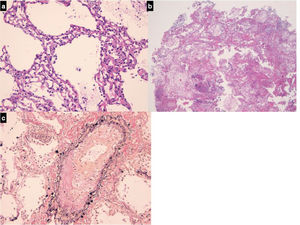
Histopathologic findings. In both cases visceral pleura was detected in biopsy samples. It appeared thickened and fibrotic and hyperplastic mesothelial cells were detected. The interlobular septa were thickened because of edema and fibrosis. The pulmonary veins embedded in the fibrotic interlobular septa presented partial or complete obliteration of the lumen by organized thrombi. The alveolar spaces, mainly around interlobular septa were occupied by hemosiderin-laden macrophages and the alveolar walls were expanded by dilated alveolar capillaries superimposed in rows (capillary hemangiomatosis-like aspects). Areas of parenchymal ischemic necrosis were also evident (alveolar walls identified as “ghost of the normal structures”; alveolar spaces containing proteinaceous edema and hemosiderin laden macrophages) (Fig. 2).
(a) Alveolar walls thickened containing dilated capillary superimposed in rows (H&E, mid power, x10); (b) A sample containing necrotic lung parenchyma. Lung structures are still identifiable as «ghosts», a typical effect of ischemia; intra-alveolar hemorrhage and proteinaceous edema are also evident (H&E, low power, x4); (c) in interlobular pulmonary vein the lumen is partially obliterated by an organizing thrombus (Van Gieson-elastic fibers, high power, x20).
Pathologic-radiologic correlations. The histological background here reported allows clear interpretation of the imaging findings. Alveolar consolidation and tiny cavitation are due to the necrosis together with alveolar hemorrhagic and intra-alveolar proteinaceous edema, while alveolar hemorrhage outside the areas of necrosis is mainly the cause of the ground glass attenuation. The capillary hemangiomatosis-like aspects are the morphological basis of the “crazy paving pattern”. Finally, the thickening of the interlobular septa visible in CT scans are the radiological manifestation of chronic venous thrombosis and septal edema and fibrosis.
Like previous reports, in our case series the clinical features of this dramatic complication also became manifest at around 12 months from the RF ablation.8 In conclusion the histopathologic findings detected in pulmonary and pleural biopsies explain the CT aspects of PVS as a complication of transcatheter RF ablation. These imaging features are similar to those observed in idiopathic veno-occlusive disease or pulmonary capillary hemangiomatosis. However, the presence of ischemic areas and infarction necrosis on biopsy samples are not observed in the above mentioned primary vascular disorders. The parenchymal venous infarcts are, along with the lobar or sub-lobar distribution of the lesions, the most relevant cue for hypothesizing the diagnosis. The presence of organized thrombi in the peripheral pulmonary veins confirm that the intravascular damage starts in the larger veins and subsequently extends to the peripheral venules and this could explain why in some cases an irreversible pulmonary hypertension may complicate the disorder when not treated early.4-6
We acknowledge Foschini Thomas MEng for English language editing.