Churg–Strauss syndrome (CSS) is a systemic necrotizing vasculitis of the small and medium vessels, associated with extravascular eosinophilic granulomas, peripheral eosinophilia and asthma.
This is a rare syndrome of unknown etiology, affecting both genders and all age groups.
CSS patients usually respond well to steroid treatment, although relapses are common after it ends. Timely diagnosis and treatment generally lead to a good prognosis with a 90% survival rate at one year.
A brief review of CSS is presented, with particular attention to diagnosis, therapy and recent developments in this area.
The authors then report and discuss the clinical, laboratory and imaging characteristics of four patients admitted to an Internal Medicine Department with this diagnosis. The treatment, response and follow-up of the cases are also described.
A síndrome de Churg-Strauss (SCS) é uma vasculite sistémica necrotizante, que afeta os vasos de pequeno e médio calibre e se associa a granulomas eosinofílicos extravasculares, eosinofilia periférica e asma.
É uma síndrome rara, de etiologia desconhecida e que afeta ambos os géneros e todos os grupos etários.
Os doentes com SCS geralmente apresentam boa resposta à terapêutica com glucocorticoides, embora as recidivas sejam frequentes após a sua suspensão. O diagnóstico e terapêutica atempada levam geralmente a um bom prognóstico, com uma sobrevivência de 90% um ano após o diagnóstico.
Neste artigo é apresentada uma breve revisão da SCS, com particular atenção ao diagnóstico, terapêutica e progressos recentes nesta área.
De seguida, os autores apresentam e discutem as características clínicas, laboratoriais e imagiológicas de quatro doentes internados num Serviço de Medicina Interna com este diagnóstico. O tratamento instituído, as respostas observadas e o seguimento dos casos são também descritos.
Churg–Strauss syndrome (CSS), also called granulomatous and allergic angiitis is a necrotizing systemic vasculitis, which was first described in 1951 by the pathologists J. Churg and L. Strauss in an autopsy series of patients with severe asthma.1 These patients had hypereosinophilia and systemic vasculitis with the histological examination showing extravascular granulomas, tissue eosinophilia and necrotizing vasculitis.
Further case reports led to an improvement in the definition; the American College of Rheumatology (ACR) classification criteria in patients with biopsy proven vasculitis is the most widely used today.2 These criteria include asthma, greater than 10% peripheral eosinophilia, mono or polyneuropathy, migratory pulmonary infiltrates, sinus anomalies and extravascular eosinophils in the biopsy. The presence of four of these criteria in a patient with biopsy proven vasculitis has a sensitivity of 85% and a specificity of 99.7% for CSS.2
CSS is a very rare disease, with an incidence of 1–7 cases per million per year.3,4 The disease affects both genders equally and can present at any age, with an average age of 50 at presentation.5,6
EtiologyThe etiology of CSS is unknown. Proposed hypotheses include an auto-immune process, possibly associated with genetic and environmental factors.7
In the last decade, the leukotriene receptor antagonists (LTRAs), including zafirlukast and montelukast have been reported to be associated with the development of CSS. This is probably related to steroid withdrawal after starting LTRAs in patients with hitherto attenuated CSS or the prescription of LTRAs to patients with worsening asthma which was actually unrecognized worsening CSS.8,9 In fact there have been reports of CSS after starting inhaled steroids10 and omalizumab11 and a recent case crossover study showed that several asthma control medications are associated with the onset of CSS, supporting the hypothesis that an aggravation of asthma is associated with CSS onset, rather than a specific drug.12
PathophysiologyThe pathophysiology of CSS is not sufficiently well known. A particular feature of the disease is the prominent blood and tissue eosinophilia which is related to the activity of the disease.13 In patients with active disease, eosinophils express surface markers of activation14 and secrete proteins that lead to tissue injury.15 The eosinophilia seems to be maintained by increased release of IL-5, produced mainly by activated Th2 lymphocytes.16 In effect, the anti-IL5 antibody mepolizumab turned out to be effective in refractory CSS in a recent study.17 Although CSS is mainly associated with a Th2 profile, recent studies have also shown possible imbalances in the activity of Th1,18 T regulatory19 and Th17 lymphocyte lineages.20 Anti-neutrophil cytoplasmic antibodies (ANCAs), specifically anti-myeloperoxidase antibodies seem to have a pathogenic role in the vasculitic lesions, possibly through the activation of neutrophils leading to the release of reactive oxygen species and proteolytic enzymes.21 The proposed roles of eosinophils and ANCAs in disease pathogenesis are in agreement with the recent reports of different disease manifestations in ANCA-positive and ANCA-negative patients.
Clinical manifestationsThe clinical manifestations of CSS are generally described in three stages.
The first stage (prodromal stage) can last for several years. Atopic asthma and rhinitis are the predominant features, usually difficult to control.
In the second stage (eosinophilic stage), an intense tissue infiltration by eosinophils is seen in various organs, including the lungs and gastro-intestinal (GI) tract, eventually evolving into Loeffler's syndrome or eosinophilic gastroenteritis.
The third (vasculitic) stage of the disease is characterized by an intense systemic vasculitis, with general symptoms usually predating the involvement of various organs by this process.22
One of the systems that is frequently affected by the vasculitis is the peripheral nervous system, with involvement of the vasa vasorum and development of mononeuritis multiplex.23 The GI tract is also affected, with abdominal pain, gastroenteritis or even full-blown acute abdomen as symptoms.24 Cardiac involvement can present as myocarditis, heart failure, pericarditis, or even coronary vasculitis and consequent myocardial ischemia. About half of all the CSS associated deaths are caused by cardiac lesions.25The respiratory system is involved in virtually all patients, not only with asthma in the first clinical stage, but also with infiltrates in the chest X-ray, that are present in 37–77% of patients. Pleural effusion can also be seen in up to a third of the patients.26–28
Clinically significant renal disease is both less frequent and serious in CSS than in other types of vasculitis.24
Disease subtypesRecent studies have demonstrated the existence of two discrete CSS patient populations. ANCA positive patients (40% in these studies) present more frequently with vasculitis related manifestations, including renal and peripheral nervous system involvement as well as alveolar hemorrhage, purpura and vasculitis in the biopsy. ANCA negative patients more frequently have manifestations related to eosinophil infiltration, including cardiac, pulmonary and systemic symptoms. It is still unclear how relevant these new findings are for the therapy and prognosis of CSS.29
Laboratory findings and diagnosisThe diagnosis of CSS is generally dependent on histological analysis, although this may not be needed if the patient has eosinophilia and the typical clinical manifestations. The most frequent tissue samples come from the GI tract (eosinophilic enterocolitis with necrotizing or granulomatous vasculitis), and from the skin, where several patterns of lesion can be seen (even in the same patient), including extravascular granulomas, leukocytoclastic vasculitis and cutaneous polyarteritis nodosa.
Nerve biopsies frequently demonstrate necrotizing epineural vasculitis. The lung is also frequently sampled, revealing patterns of bronchial asthma, eosinophilic pneumonia and extravascular granulomatosis.30–32
The two most suggestive and frequent histological findings of CSS are necrotizing vasculitis and extravascular necrotizing granulomas, with eosinophilic infiltrates. These findings, however, are not pathognomonic.33
Blood tests show a significant increase in inflammation markers and intense peripheral eosinophilia. The titers of IgE and rheumatoid factor are also frequently elevated. ANCAs are typically present (40–70% of patients), showing a perinuclear pattern in almost all cases (p-ANCAs).5
The differential diagnosis includes hypereosinophilic idiopathic syndrome, chronic eosinophilic pneumonia and various other forms of systemic vasculitis.5,34,35
TreatmentThe treatment of CSS is similar to various other small-vessel vasculitis. High dose steroids are the mainstay of treatment and generally begin with a dose of 1mg/Kg/day (max 60mg/day) of prednisolone, maintained until symptoms are controlled and then slowly tapered (for one year). In patients with factors which have a poor prognosis (central nervous system, renal, cardiac or gastro-intestinal involvement), the combination of steroids and cyclophosphamide produced better control and sustained remission rates,6 compared to steroids alone and is therefore included for remission induction in the European League Against Rheumatism (EULAR) recommendations for the management of small and medium vessel vasculitis. In non-organ threatening or non-life threatening disease, the same document recommends the use of methotrexate as a less toxic alternative to cyclophosphamide. For remission maintenance, the combination of low-dose steroids and azathioprine, leflunomide or methotrexate is used.36
Patients not responding or who relapse despite appropriate therapy are managed with different therapies, including mycophenolate mofetil,37 human intravenous immunoglobulin,38 and biological agents including rituximab39, interferon-alpha,40,41 and mepolizumab.17,42
PrognosisIn the absence of treatment, CSS can be rapidly fatal after vasculitis has set in. Treatment with steroids leads to a significant response in 90% of patients, although about 20% will need additional cytotoxic therapy.
Treatment dramatically changes the prognosis of CSS patients (over 90% survival in one year). Nevertheless, following suspension of steroids, recurrent disease is observed in up to 25% of patients in the first five years, with asthma and sinusitis being the most frequent manifestations.43 Most recurrences show a good response to steroids. The leading cause of chronic morbidity in patients with CSS is neurological disease.37,43
Mortality in CSS patients is usually the result of cardiac or GI involvement, with myocarditis, heart failure, pericarditis, or even coronary vasculitis in the former and hemorrhage, perforation or entero-colic necrosis in the latter. Hemorrhagic stroke can also be a cause of death.
Through the analysis of a large number of CSS cases, Guillevin et al. recognized five factors associated with a worse prognosis, if they are present at the time of diagnosis (cardiomyopathy, CNS involvement, severe GI disease, renal failure with creatinine over 1.58mg/dl and proteinuria over 1g/day).44
MethodsAll patients admitted to the Department of Internal Medicine during a 24-year period fulfilling ACR diagnosis criteria for CSS were included. The clinical laboratory, imaging and follow-up data was attained through the analysis of the clinical files.
ResultsPatientsFour patients were included (2M/2F), with an average age of 48 years (34–60) at diagnosis.
Case summariesPatient 1A 46-year-old female patient presented with weight loss, paresthesia, myalgia, arthralgia and loss of muscular strength in her lower limbs in the previous month, and abdominal pain, diarrhea, fever and night sweats in the last four days. She also complained of productive cough and worsening dyspnea in the last few days. Past medical history included asthma and sinusitis in the last two years. Physical examination revealed maculo-papular lesions in the left foot, generalized hyporreflexia, and loss of muscular strength in the lower limbs. Blood tests showed marked eosinophilia and skin biopsy showed signs of vasculitis with eosinophils in vessel walls. Treatment with high dose oral prednisolone and cyclophosphamide led to a rapid response and the patient remained asymptomatic. Three years later she had a relapse and was treated with the same regimen, with good results.
Patient 2A 34-year-old male patient was admitted due to complaints of asthenia, anorexia, diffuse myalgia and weight loss in the last three weeks. He also complained of dyspnea, cough with sputum and skin lesions in the lower limbs. Medical history was significant for asthma and sinus disease with polyposis for 4 years. Physical exam was compatible with polyneuropathy. Blood tests revealed significant eosinophilia and chest CT revealed a pulmonary infiltrate. Skin biopsy revealed aspects of Churg–Strauss granuloma and extravascular eosinophils caused. There was a rapid and significant improvement after treatment with high dose prednisolone and the patient remains on follow-up for controlled asthma.
Patient 3A 60-year-old female patient presented having suffered from pain and paresthesia in the left hand and right leg for the previous week, and abdominal pain and diarrhea for two months. She had been diagnosed with asthma and rhinitis for 9 years. On neurological exam she displayed ataxia and loss of muscular strength in the lower limbs. Blood tests revealed marked eosinophilia and chest imaging showed migratory pulmonary infiltrates. Biopsy of colonic mucosa was compatible with eosinophilic colitis. She was treated with prednisolone and gabapentine, and later IV cyclophosphamide but maintained significant neurological complaints. This led to the use of IV human immunoglobulin with improvement after 6 cycles.
Patient 4A 53-year-old male admitted for a 4-month history of cutaneous vasculitis in the lower limbs, and abdominal pain and diarrhea in the previous month. Past medical history included asthma for three years, alcohol abuse until 18 months earlier and dilated myocardiopathy with severe heart failure for two years. Physical exam showed hyporreflexia, hypoesthesia and lower limbs petechiae. Blood tests revealed eosinophilia and sinus CT showed signs of sinusitis. Biopsy of colonic mucosa was compatible with eosinophilic colitis and skin biopsy showed eosinophilic vasculitis. Treatment with prednisolone caused rapid improvement of all complaints, but the patient died two weeks after release, of undetermined cause.
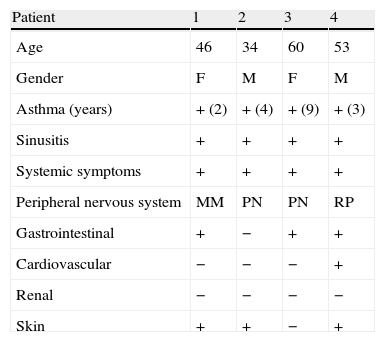
Description of case seriesClinical characteristicsThe patients’ clinical characteristics are summarized in Table 1.
Patients clinical characteristics at the time of diagnosis.
| Patient | 1 | 2 | 3 | 4 |
| Age | 46 | 34 | 60 | 53 |
| Gender | F | M | F | M |
| Asthma (years) | + (2) | + (4) | + (9) | + (3) |
| Sinusitis | + | + | + | + |
| Systemic symptoms | + | + | + | + |
| Peripheral nervous system | MM | PN | PN | RP |
| Gastrointestinal | + | − | + | + |
| Cardiovascular | − | − | − | + |
| Renal | − | − | − | − |
| Skin | + | + | − | + |
MM: mononeuritis multiplex, PN: polyneuropathy, RP: radiculopathy.
All patients had systemic, pulmonary and nervous system involvement. Systemic symptoms included fever, weight loss and diffuse myalgia. All patients had asthma, and two also had complaints of worsening dyspnea and productive cough. Although only two patients complained of paresthesia, on examination all had neurological abnormalities, including muscle weakness in two patients and hypoesthesia in all four. The GI tract was affected in three patients, with diarrhea and abdominal pain.
Skin changes were found in three patients (signs of vasculitis in the lower limbs).
Only one patient had cardiac involvement, which was most likely prior to CSS, since it had started before all symptoms except for asthma and was probably associated with chronic alcohol abuse. No renal, ophthalmic or joint abnormalities were reported in any patient. Neither was CNS involvement described.
Past medical historyPast medical history was significant for previously diagnosed asthma in all patients, two of them medicated with LTRAs. One patient also had dilated myocardiopathy with severe heart failure, probably due to chronic alcohol abuse.
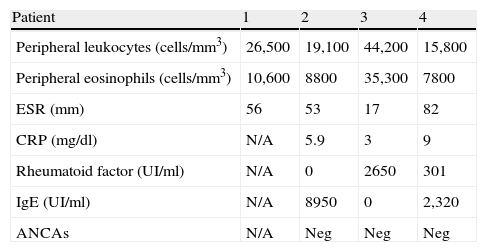
Laboratory abnormalitiesThe summary of laboratory characteristics of all patients is presented in Table 2.
Patients laboratory characteristics.
| Patient | 1 | 2 | 3 | 4 |
| Peripheral leukocytes (cells/mm3) | 26,500 | 19,100 | 44,200 | 15,800 |
| Peripheral eosinophils (cells/mm3) | 10,600 | 8800 | 35,300 | 7800 |
| ESR (mm) | 56 | 53 | 17 | 82 |
| CRP (mg/dl) | N/A | 5.9 | 3 | 9 |
| Rheumatoid factor (UI/ml) | N/A | 0 | 2650 | 301 |
| IgE (UI/ml) | N/A | 8950 | 0 | 2,320 |
| ANCAs | N/A | Neg | Neg | Neg |
ESR: erythrocyte sedimentation rate, CRP: C reactive protein−normal<0.5, IgE: total immunoglobulin E, N/A: not available.
All patients had leukocytosis and eosinophilia at the time of diagnosis, with the peripheral blood eosinophils varying between 7800 and 35,000G/L. Erythrocyte sedimentation rate was elevated in three patients. The levels of IgE were quantified in three patients and were elevated in two of them. Three patients were tested for ANCAs, but none was positive.
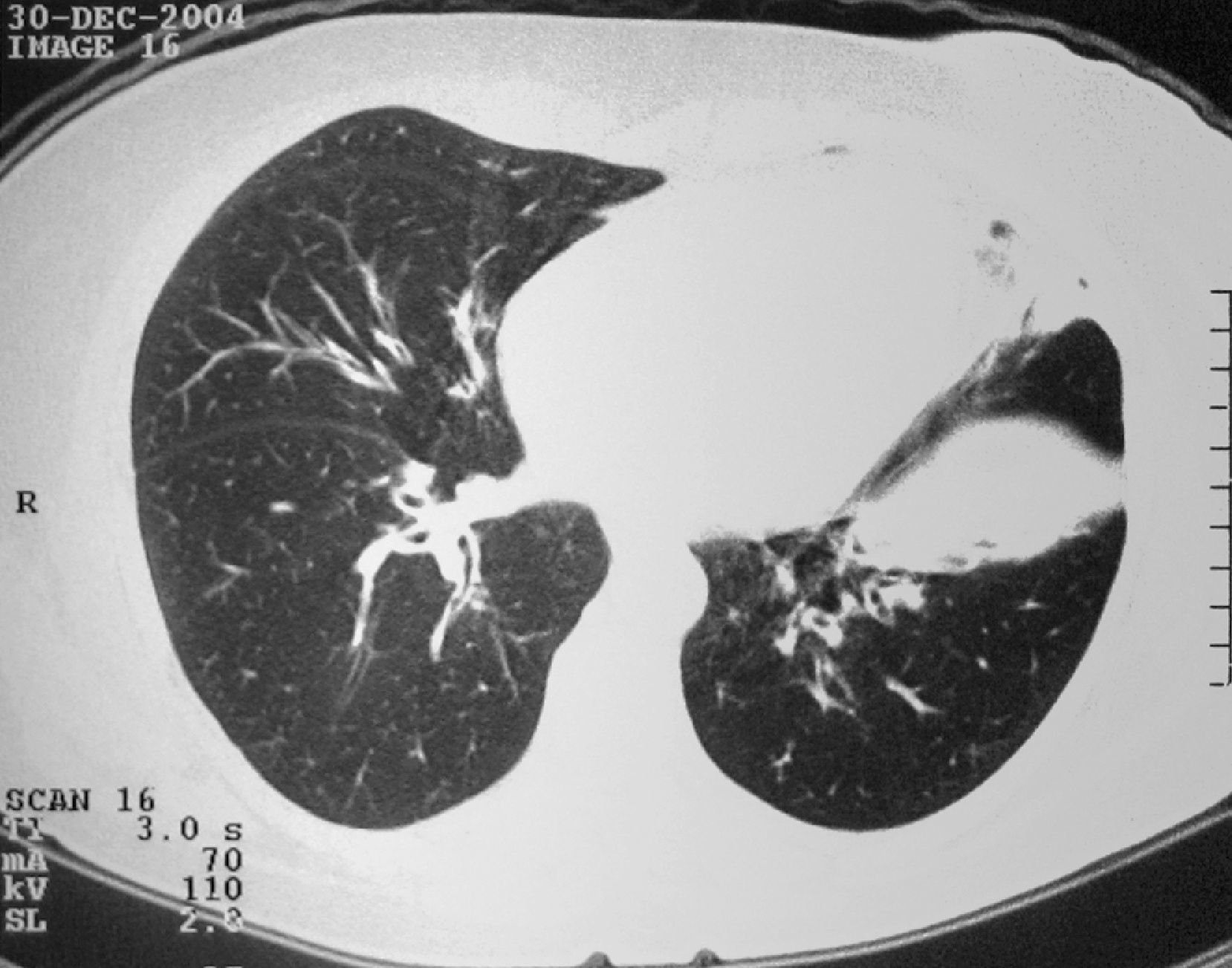
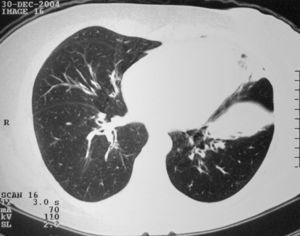
Three of the patients had relevant changes in the chest X-ray, although only one showing migratory infiltrates generally associated with CSS. Chest CT was performed in three patients revealing abnormalities in both the interstitium and the large bronchi (Fig. 1).
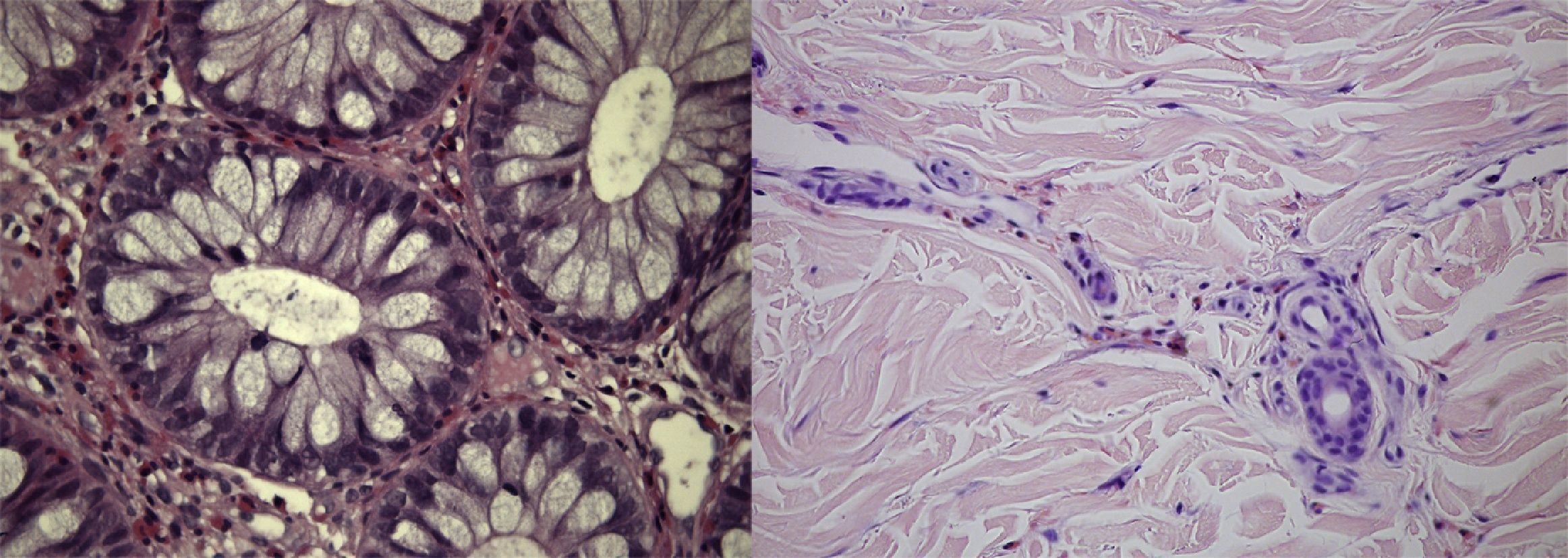
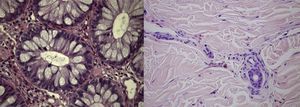
Histological analysisAll patients had a histological confirmation of the vasculitis diagnosis. Two had skin biopsy, one had colonic mucosal biopsy and the fourth had both skin and colonic mucosal biopsies. Biopsies of the colonic mucosa showed aspects of active eosinophilic colitis (Fig. 2) and skin biopsies revealed aspects of eosinophilic vasculitis in two patients and typical aspects of the Churg–Strauss granuloma in another.
TreatmentAll patients received treatment with high doses of systemic steroids (≥1mg/kg/day of prednisolone or equivalent).
An immediate and relevant response to the treatment was seen in all patients, with improvement in all but the neurological complaints within two to four days. Troublesome neurological symptoms required the addition of another drug in three patients, namely cyclophosphamide in one case, gabapentine in another and gabapentine and cyclophosphamide, followed by gabapentine and human immunoglobulin in another.
Follow-upNone of the patients died while in hospital. Three were free of symptoms when discharged from hospital, 12–46 days after admission. The other patient continued to suffer from serious neurological complaints (upper limb paresthesia with loss of function).
One of the patients disappeared from follow-up after 3 years. Another died two weeks after discharge, at home, probably due to worsening severe chronic heart failure secondary to dilated myocardiopathy which had been present before diagnosis of CSS. The other two patients are still in follow-up; one of them (patient 3) still suffers from asthma and intense upper member paresthesia which only improved after 6 cycles of endovenous human immunoglobulin. The other patient (patient 2) only suffers from asthma.
RelapsesOnly one relapse was recorded, in patient 1, three years after diagnosis, characterized by complaints of polyarthralgias and cutaneous lesions, and leukocytosis with eosinophilia, which all responded well to treatment with prednisolone and cyclophosphamide.
DiscussionThe demographic and clinical characteristics of our patients are similar to those in previously published series.45–47
The fact that two of the patients were taking LTRAs before the diagnosis of CSS is not particularly relevant, since these drugs are frequently prescribed to patients suffering from atopic asthma, as they are to patients with CSS. A detailed discussion of the association between LTRAs and CSS is beyond the scope of this article and can be found elsewhere.9
It is important however to underline the high rate of neurological involvement in our patients, although only one presented the classical finding of mononeuritis multiplex. Radiculopathy has already been described in a patient with CSS, but seems to be a rare finding.48
It is interesting that none of blood tests of the three patients were positive for ANCA, unlike in most published series. This is probably due to the small number of patients in our case series.
The difficulty in treatment of neurological complications is also relevant, although this has also been discussed in other series. The good results obtained with the use of immunoglobulin, after failure of steroids, gabapentine and cyclophosphamide have been previously described.49
Although extensive follow-up information was only available for two patients, the absence of mortality during hospital admission is consistent with the good prognosis reported in the literature for CSS, where there is timely and correct diagnosis and therapy. Although one patient died 2 weeks after discharge, this was probably due to his existing heart condition.
In conclusion, CSS is a rare but easily diagnosed disease if there is a high level of clinical suspicion. It is important that all clinicians dealing with patients with asthma or vasculitis are aware of the clinical imaging and laboratory characteristics of this disease, as timely and appropriate treatment has significant impact on both quality of life and survival.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Alfaro TM. Síndrome de Churg-Strauss: casuística. Rev Port Pneumol. 2012; doi:10.1016/j.rppneu.2011.12.001