Nontuberculous mycobacteria (NTM) are opportunistic agents that have gained importance during the last decades due to their increasing incidence in high-risk populations. Their modes of transmission differ from person-toperson contact commonly described in Mycobacterium tuberculosis (MTB). In fact, NTM are frequently found in soil, natural waters and drinking-water distributions systems, emphasizing the contribution of environmental factors when discussing this disease’s susceptibility. Our aim is to evaluate the incidence of NTM in Portugal and to identify the main environmental variables related to it.
Material and MethodsWe performed a cross-sectional study centred on 2011 (date of the latest Portuguese census) from collected personal features and environmental data available in public databases. Environmental values when only known at the district level were interpolated using inverse distance weighting. A semiparametric poisson model was used to estimate NTM incidence. The non-parametric part of the model was obtained by using thin plate smoothing splines defined on the spatial component of the data.
Results359 new NTM cases were notified during a five-year period. None of the environmental determinants studied was strong enough to predict NTM geographical incidence in Portugal (p>0.05), except for population density (p<0.001). Personal characteristics such as female sex (p<0.001), age (p<0.001) and Human Immunodeficiency Virus infection and Acquired Immune Deficiency Syndrome (HIV/AIDS) incidence (p<0.001) are associated with an increase of NTM disease incidence.
ConclusionsNTM appears to be more common in elderly women, especially if they have HIV/AIDS disease or if they live in urban, highly populated areas. Overall, female sex seems to assume the most relevant role when discussing predisposition to NTM disease. However, further studies are needed to evaluate the impact on NTM geographical incidence by other environmental and personal variables not included in this one.
Nontuberculous mycobacteria (NTM) are opportunistic human pathogens known to be an important cause of morbidity and mortality in immunocompromised individuals.1 NTM epidemics have changed since the emergence of Human Immunodeficiency Virus infection and Acquired Immune Deficiency Syndrome (HIV/AIDS), transforming this rare infection into a common diagnosis in high-risk populations.2–4 Despite still being underdiagnosed, NTM prevalence has exponentially grown during recent decades and it will continue to increase given the greater life expectancy and the lengthened survival of risk groups in developed countries.1,5,6
It is now known whether NTM are transmitted via aerosols.2,4–6 A great influencer in NTM epidemiology is its lipid-rich outer membrane constituted by long chain mycolic acids which makes them more impermeable with a slower growth rate.6,7 The characteristics of this membrane explain not only the preferential attachment to surfaces but also why NTM are so widespread despite low rate division and impenetrability to hydrophobic nutrients.2,5,6,8
Originally, NTM were identified in natural waters and soils and unusual places such as acid, brown water swaps, boreal and peat rich soils and metal working fluids.2,6 These findings show that NTM are able to thrive in a diverse range of organic compounds but mostly acidic waters and soils and areas with air:water interfaces; they also prove that its growth is not influenced by temperature range, degree of salinity or oxygen tension.2,6,8,9 In addition to their presence in natural environments, NTM are frequently isolated from household plumbing, drinking-water distribution systems and hospital water systems where they proliferate as a biofilm which contributes to their intrinsic resistance to pollutants and heavy metals.2,5–8 Also, human activities such as disinfection, promotion of polluted environments and injudicious use of antibiotics help to select bacteria progressively more resistant and accustomed to human environments, specially urban areas and most densely populated places.2,6,8
Worldwide, NTM appear to be more frequent in tropical and subtropical regions; however, it is now recognized that epidemiological data varies according to geographical areas which in turn correlates with local clinical settings.8,10 So, it is necessary to conduct epidemiological surveillance in order to provide local epidemiological data that is useful in the management of patients.10 In this study, we will evaluate the incidence on NTM in Portugal and its association with some environmental variables (temperature, soil, pH, humidity and precipitation).
Material and methodsWe conducted a cross-sectional study centred on 2011 (date of the latest nationwide census performed in Portugal).
The new NTM cases reported between 2009 and 2013 were extracted from Sistema de Vigilância Intrínseco do Programa Nacional de Luta Contra a Tuberculose (SVIG-TB),11 a public health surveillance system for tuberculosis cases in Portugal which also includes nontuberculous mycobacteria data (i.e. a database for both tuberculous and nontuberculous mycobacteria). Population density data and personal features of the Portuguese population such as age, sex, HIV/AIDS incidence and migrants' proportion were collected from the 2011 nationwide census organized by Instituto National de Estatística (INE),12 an institute responsible for producing official statistical data for the Portuguese government. Environmental determinants such as temperature, humidity and precipitation in the period between 2009 and 2013 were obtained from Instituto Português do Mar e da Atmosfera (IPMA),13 a public institute with nationwide research responsibilities concerning the ocean and the atmosphere. Soil pH data were collected from the European Soil Data Centre (ESDAC) dataset,14,15 a project from the Joint Research Centre (JRC) organised by the European Commission.16 To simplify our geographical analyses, we excluded the archipelagos of Azores and Madeira (Azores and Madeira inhabitants represent approximately 5% of the total Portuguese population) and considered the 278 Portuguese municipalities as the spatial reference units. All data were collected according to this geographical division, except for IPMA’s data which were only available for districts.
Microsoft EXCEL (2013) and R (version 3.5.2) were the data analyses tools used. Values collected from IPMA are monthly averages over a 5-year period and were interpolated using the inverse distance weighting method in order to obtain values for each municipality. Semiparametric poisson models were used to estimate NTM incidence. The non-parametric part of the models was obtained by using thin plate smoothing splines defined on the spatial component of the data. All models include the spatial component so that each explanatory variable is adjusted for geographical localization. Selection of variables in order to obtain a final model was made using the backward stepwise method. The level of significance was set at a p<0.05.
ResultsAccording to 2011 nationwide census, 10,562,178 people lived in Portugal during that year. Between 2009 and 2013, 359 new NTM cases were reported to SVIG-TB (mean=71.8 cases/year). The majority came from Lisbon (mean=8.0 cases/year) and Oporto (mean=6.8 cases/year) municipalities, the most populated areas in Portugal.
As for the environmental variables, we found a positive effect of population density on the NTM geographical incidence (Expected effect (EE)=1.123, CI=1.054–1.198). Humidity (EE=1.21, CI=0.802–1.825) and precipitation (EE=1.044, CI=0.964–1.130) had the same effect but in a smaller magnitude. Conversely, mean temperature (EE=0.399, CI=0.077–2.076) and pH (EE=0.859, CI=0.575–1.283) were associated with a negative effect on NTM geographical incidence. Except for population density, none of those associations were statistically significant (p>0.05) (Table 1).
Effect of each environmental variable adjusted to NTM geographic distribution.
| Environmental determinants | Expected effect (EE) | Confidence Interval [95% CI] | p Value |
|---|---|---|---|
| Humidity (%) | 1.210 | 0.802–1.825 | 0.363 |
| Mean temperature (ºC) | 0.399 | 0.077–2.076 | 0.275 |
| pH | 0.859 | 0.575–1.283 | 0.457 |
| Population density (thousands/km2) | 1.123 | 1.054–1.198 | <0.001 |
| Precipitation (mm or kg/m2) | 1.044 | 0.964–1.130 | 0.288 |
The value of expected effect translates how much more effect that variable will have on NTM geographical incidence. For example, considering humidity, for each increase of one percent in humidity there will be a 21% increase in expected NTM incidence. However, this increase does not inform us about the impact of this variable on NTM geographical incidence. For that, we must analyse the confidence interval from narrowest and farthest intervals of 1 translated into a greater overall impact on NTM incidence.
Note: For each environmental determinant the mean values from period 2009–2013 was considered, except for population density and pH.
Regarding personal features, sex has shown to have the greatest effect on NTM geographical incidence (EE=0.695, CI=0.612-0.788), in that male sex appears to act as a protective factor on NTM infection. Age older than 45 years old had the second greatest effect on NTM geographical incidence (EE=1.091, CI=1.049–1.134). HIV/AIDS incidence (EE=1.019, CI=1.010–1.028) was also positively associated to NTM geographical incidence. The migrant proportion was another variable positively related to NTM geographical incidence but not as strong as others (EE=1.095, CI=1.029–1.164) (Table 2).
Effect of each personal feature adjusted to NTM geographic distribution.
| Personal features | Expected effect (EE) | Confidence Interval [95% CI] | p Value |
|---|---|---|---|
| Age >45 years old | 1.091 | 1.049–1.134 | <0.001 |
| HIV/AIDS incidence (per 100.000hab) | 1.019 | 1.010–1.028 | <0.001 |
| Male sex (%) | 0.695 | 0.612–0.788 | <0.001 |
| Proportion of migrants (per 1000hab) | 1.095 | 1.029–1.164 | 0.004 |
The value of expected effect translates how much more effect that variable will have on NTM geographical incidence. For example, considering age older than 45 years, for an increase of a percentage point in the proportion of older people in the population there will be a 9.1% increase in expected NTM incidence. However, this increase does not inform us of the impact of this variable on NTM geographical incidence. For that, we must analyse the confidence interval since narrowest and farthest intervals of 1 translate into a greater overall impact on NTM incidence.
Note: For each personal feature, values from the 2011 portuguese census were considered.
We studied all patients notified with NTM in a period of 5 years (n=359) and calculated an annual incidence of 71.8 cases/year. Of all environmental determinants analysed, population density (p<0.001) is the only environmental variable that has been shown to influence NTM epidemiology. Personal characteristics such as female sex (p<0.001), age (p<0.001) and HIV/AIDS incidence (p<0.001) demonstrated a positive effect on the NTM geographical incidence.
Cassidy et al. described the microbiologic and demographic features of NTM disease when estimating the population-based prevalence of pulmonary and extrapulmonary NTM cases in Oregon. They were capable of associating the higher prevalence of disease in the western, more urban portion of Oregon to a wetter and more temperate climate relative to what was observed in rural areas of the same state.1 In a study conducted by Iivanainen et al., to evaluate the impact of environmental factors on the occurrence of environmental mycobacteria, they found that the number of total NTM in Finnish brook waters also correlated positively with precipitation.2,17 They proved that in rainy periods there is an increase in chemical oxygen demand, acidity and counts of mycobacteria in the waters.17
In our population, we also found a positive effect of humidity and precipitation and a negative one by pH and temperature on NTM geographical incidence, albeit not being statically significant. In part, these results can be explained by our small sample size.
We found, as well, that the population density positively influences NTM incidence. Cassidy et al. also confirmed this when they studied NTM cases in Oregon residents.1 They found a higher prevalence of disease in the western, more urban portion of Oregon that they attributed to the use of municipal water systems, since it had already been demonstrated in urban communities of Boston in 1970s/80s that water stored in reservoirs for long periods of time promotes biofilm formation.1,18,19 However, as Cassidy et al. admitted, it is possible that these results are influenced by a diagnostic bias since patients with chronic lung diseases might more commonly live in urban areas and therefore more easily seek medical care.1 In addition, another diagnostic bias may be present in these patients as NTM infection is higher in individuals with chronic lung diseases.4,5,10,20,21
‘Windermere syndrome’ corresponds to a single case of an elderly, non-smoking, thin woman where pulmonary disease was for the first time attributed to NTM.1,5,22 Subsequent studies confirmed that NTM pulmonary disease is more common in previously healthy elderly women.7,9,10,19,23–25 This is similar to our findings that female sex and age greater than 45 years old are, respectively, the first and second best NTM geographical incidence influencers when analysed individually. But when creating a model starting with all significant variables together with geographic variation and applying a selection of variables procedure, sex is the only one to appear in the final model (see Figs. 1 and 2). Although Stout et al. confirmed this increase in NTM incidence with age, they argued that gender predominance in NTM could be wrongly overestimated in men by the different prevalence of smoking-associated lung damage between males and females.5,9
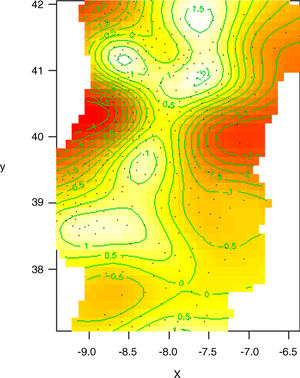
NTM incidence model using geographical distribution as a single influencer. This model does not represent actual NTM cases distribution, but it is rather predicted NTM incidence variation solely by geographical distribution. White areas represent places with highest predictable NTM incidences. Red areas represent places with lowest predictable NTM incidences. Green lines are log-scale level curves that compare predicted NTM local incidences to the mean value of NTM incidence considered for mainland Portugal.
x=latitude, y=longitude
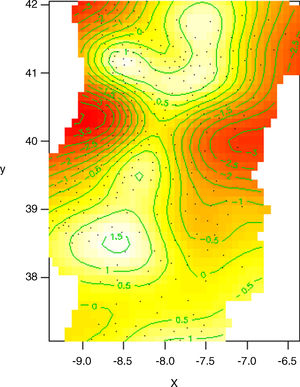
Effect of geographic distribution on NTM incidence when adjusted to male proportion. In this model, we subtract the impact of sex on NTM incidence, considering that male proportion is the same for all municipalities. By doing this, we can see how much more geographical variation influences NTM incidence (comparatively to what was already explained by sex). This model predicts better actual NTM cases distribution because it matches geographical variation with male proportion.
x=latitude, y=longitude.
Our study similarly found a positive influence of HIV/AIDS incidence on NTM geographical incidence. This agrees with the current evidence that NTM cases are highest among immunosuppressed groups.1,3–5,26 When considering the Portuguese landscape, the relationship between HIV/AIDS incidence and NTM geographical incidence was strongest in Lisbon and Oporto regions, areas where HIV/AIDS incidence is higher.27
Finally, our study still found that migrant proportion has a small effect on NTM geographical incidence. A plausible theory is that migrants usually live in suburban and urban areas of the country’s most populated cities but with worse access to health care facilities and diminished hygienic-sanitary conditions compared to the native population.1,2,6,28,29 Howsoever, the idea that NTM disease is more common in the migrant population has been debated in more recent works. In a study carried out in Canada by Hernandez-Garduno et al. to determine the risk factors for pulmonary colonization by NTM, they concluded that NTM colonization risk is not so different between Canadian-born people and foreign-born people residing in Canada for at least 10 years.30 This underlines the cumulative risk of exposure to environmental sources.
Other authors have already identified others personal features as risk factors for NTM disease. Commonly, NTM infection is greater in individuals with prior or current lung diseases, patients exposed to the aspiration of gastric contents, individuals with deformations of the chest or individuals immunosuppressed for other reasons than HIV/AIDS.4,5,9,10,20,21 Genetic causes were also implicated in the pathophysiology of NTM infection.5,7 For example, Kotilainen et al. proposed C4 deficiency as a risk factor for NTM pulmonary infection in elderly female patients.7 Other research identified defects in the interleukin-12/interferon-gamma axis in families predisposed to rare disseminated NTM disease.5,7,20,31,32 Although the available evidence is limited, there is some suggestion that tobacco and alcohol may be cofactors in the development of NTM pulmonary involvement.4–6 Lastly, race appears to play a part in NTM disease susceptibility.19,30 In their works, Bodle et al. identified a higher prevalence of NTM positive cultures in white people from a sample of patients in New York City.19 Given the large number of personal features highlighted here, it is not surprising that those were the most promising factors in our study.
The strength of this study is that it is a nationwide longitudinal study with NTM cases routinely notified by family physicians. One of the limitations associated is related to the few environmental variables analysed. This was limited by the scarce data collected by the meteorological stations that are property of IPMA. Another limitation is the fact that our study used data from 2011, so it could be argued that the conclusions found are valid only for that period and no further. Also, the small number of NTM cases identified per year made it more difficult to find any statically significant result between environmental determinants and NTM incidence. Moreover, local differences in health services assessment and inequality expertise in NTM diagnosis by regional hospitals could have negatively influenced the relations studied.5,9,21 Additionally, the mathematical approach applied to the IPMA data to derive values from districts to municipalities could similarly influence the results encountered. So, a more judicious study design should be implanted in future researches to minimize these glitches.
ConclusionAlthough we had found some correlations between environmental determinants and NTM geographical incidence in mainland Portugal, population density is the only environmental variable significantly associated to the incidence of NTM. However, personal variables such as sex, age and HIV/AIDS incidence were revealed to have a greater effect on this outcome. Further studies are needed with a larger sample and with inclusion of other environmental determinants not routinely measured.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial or non-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank ISPUP (Instituto de Saúde Pública da Universidade do Porto) for helping collect all data from SVIG-TB and ESDAC datasets and, importantly, for making a financial effort to acquire meteorological data from IPMA.