Stone quarry workers are considered at increased risk from tuberculosis (TB) mainly because of their prolonged exposure to silica.1 They are considered a vulnerable population group for TB in Portugal, especially in the municipalities of Penafiel and Marco de Canaveses.2,3
Understanding transmission patterns is essential for developing prevention strategies and prioritising resources.4 Whole-genome sequencing (WGS) is an important tool to identify clusters.5,6
Data from all cases of TB in stone quarry workers from 2015 to 2019, for whom M. tuberculosis (MTB)-positive specimens were available for complementary laboratory testing (n = 35, 47.9% of the eligible sample) was analysed. The data was collected by the local public health services during their routine surveillance and public health authority duties. TB clusters were identified using WGS (single nucleotide polymorphism [SNP]-based approach7), and the largest cluster identified was investigated to distinguish potential exposure settings and propose strategies to reduce transmission.
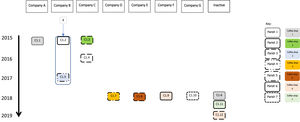
Six clusters were found. The largest cluster included 12 MTB isolates from 2015 to 2019. Five of the 12 cases (41.7%) had already had at least one previous episode of TB, six (50.0%) had silicosis and alcohol dependence, and eight (66.7%) were smokers (Fig. 1). Out of the 12 cases, who lived in seven different parishes of the Penafiel and Marco de Canaveses municipalities, nine (75.0%) were employed in seven companies, and nine (75.0%) reported frequently attending seven different coffee shops (Fig. 2).
Cases C1.2 and C1.5 were identified as close contacts of a co-worker from the same company that had had active TB in 2014 (a non-genotyped case that was considered the primary case for this cluster - X). The three worked in the central region of Portugal during the week and returned to three different parishes for the weekend. C1.2 shared the same room, and C1.5 shared transport and meals with the primary case. C1.2 had active TB in 2015, diagnosed during screening; C1.5 only had active TB in 2017 (a reinfection, previous treatment in 1999). C1.5 was identified for screening but the follow-up cannot be traced, and the screening result is not known. Failure to attend or incomplete screening was previously reported in this population,2 with active TB cases identified late among those considered close contacts.
Case C1.5, who also had silicosis and was a smoker, had a cough for three months before the diagnosis (Fig. 1); he was the suspected primary case of a community outbreak that included another six cases of TB in the first six months of 2017 in the parish where he lives. None of the other six cases were genotyped.
The primary case of this genotyped cluster (X) was considered cured in 2015 but had a second episode of active TB in 2017 at 33 years old. The new strain was genotyped and found to be associated with a different cluster suggesting that X was re-infected two years after his cure.
Case C1.9 was working abroad at the time of diagnosis and reported attending a bar also attended by C1.12 and two previous non-genotyped cases (Fig. 2).
These 12 stone quarry workers had identical strains of Mycobacterium tuberculosis (with less than six differences in SNP), which suggests that they belong to the same chain of transmission. Nevertheless, it is probable that missing intermediary cases exist both in the general community and in other non-genotyped cases among stone quarry workers. The importance of household contact was not assessed in this study, but the epidemiological enquiry did not find any relatives who previously had TB.
Based on epidemiological investigation data, only close contacts in the workplace were identified. Social connections such as those occurring in coffee shops and not identified by the case, are difficult to find. However, these social contacts seem to be important in maintaining the ongoing active transmission in this high-risk population.
Active transmission of TB among stone quarry workers in this cluster was driven by multiple factors, including those related to occupation, but also those related to social habits. We highlight that this outbreak probably spread to different regions of the country and possibly to other countries. Stone quarry workers' vulnerability, mainly due to silicosis, probably makes them more prone to TB infection and reinfection.
To better understand TB transmission dynamics in this high-risk population and the community, it would be beneficial if all the cases occurring in the country or region were genotyped. That would allow us to understand the transmission chains and different exposure settings better.
It is paramount that the local public health services explore all possible exposure settings during the epidemiological investigation whenever a new case is notified. Strategies to protect the most vulnerable should be enhanced: not only strategies addressing individuals such as promoting screening and health literacy to recognize symptoms and decrease diagnosis delay, but also environmental level strategies such as improving the ventilation conditions of the sites at which exposure occurs.
Ethical approval for this study was obtained (Ethics Boards of the Northern Regional Health Administration, 134/2022).
No funding was obtained for this study.
We would like to acknowledge Miguel Pinto (Genomics and Bioinformatics Unit, Department of Infectious Diseases, National Institute of Health, Portugal) for the collaboration on the analysis of the genomic data, and the laboratory staff of the Innovation and Technology Unit (National Institute of Health, Portugal) for the M. tuberculosis genomes sequencing. We also would like to acknowledge the professionals of the Public Health Units of Vale do Sousa Sul (Penafiel) and Baixo Tâmega (Marco de Canaveses) who performed epidemiological enquiry to all the notified cases of TB during the 2015-2019.