To characterize and compare the in vitro transport properties of respiratory mucoid secretion in individuals with no lung disease and in stable patients with chronic obstructive pulmonary disease (COPD) and bronchiectasis.
MethodologySamples of mucus were collected from 21 volunteers presenting no lung disease who had undergone surgery, from 10 patients presenting chronic COPD, and from 16 patients with bronchiectasis. Mucociliary transport (MCT), transport by cough (SCM), and contact angle (CAM) were evaluated.
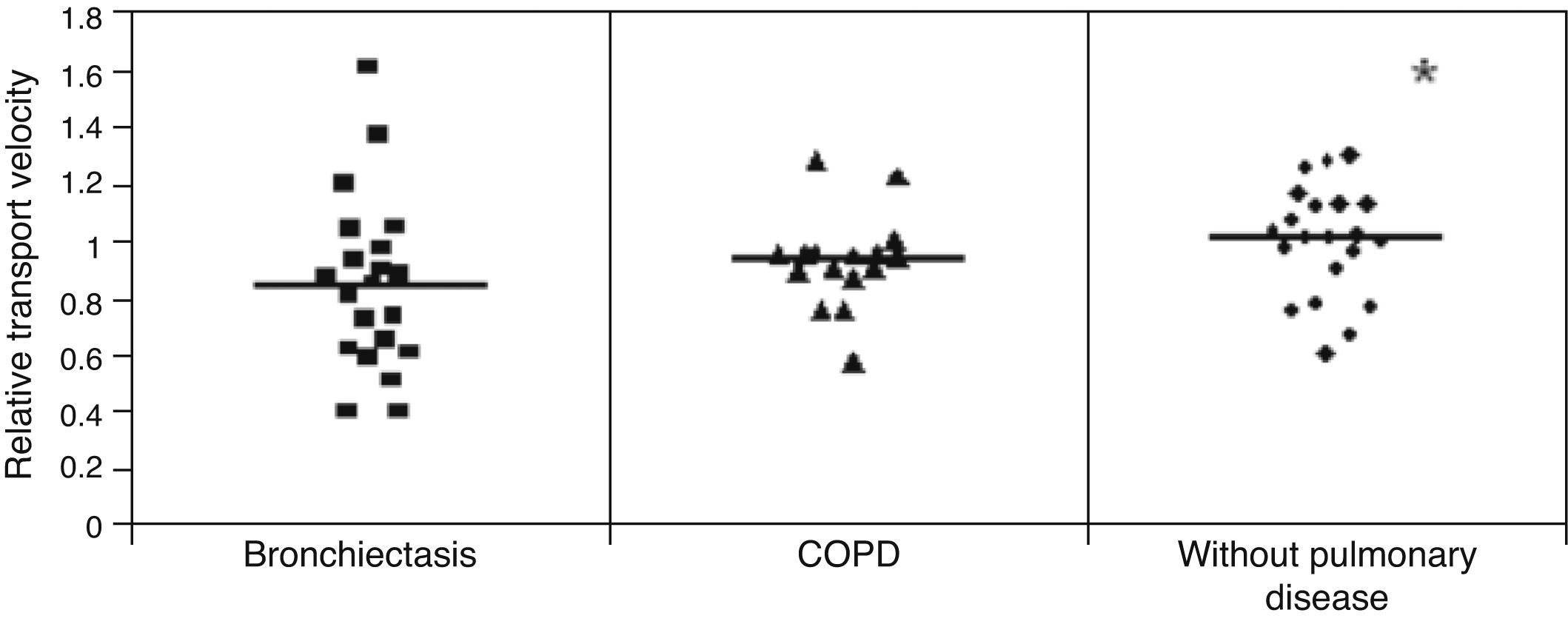
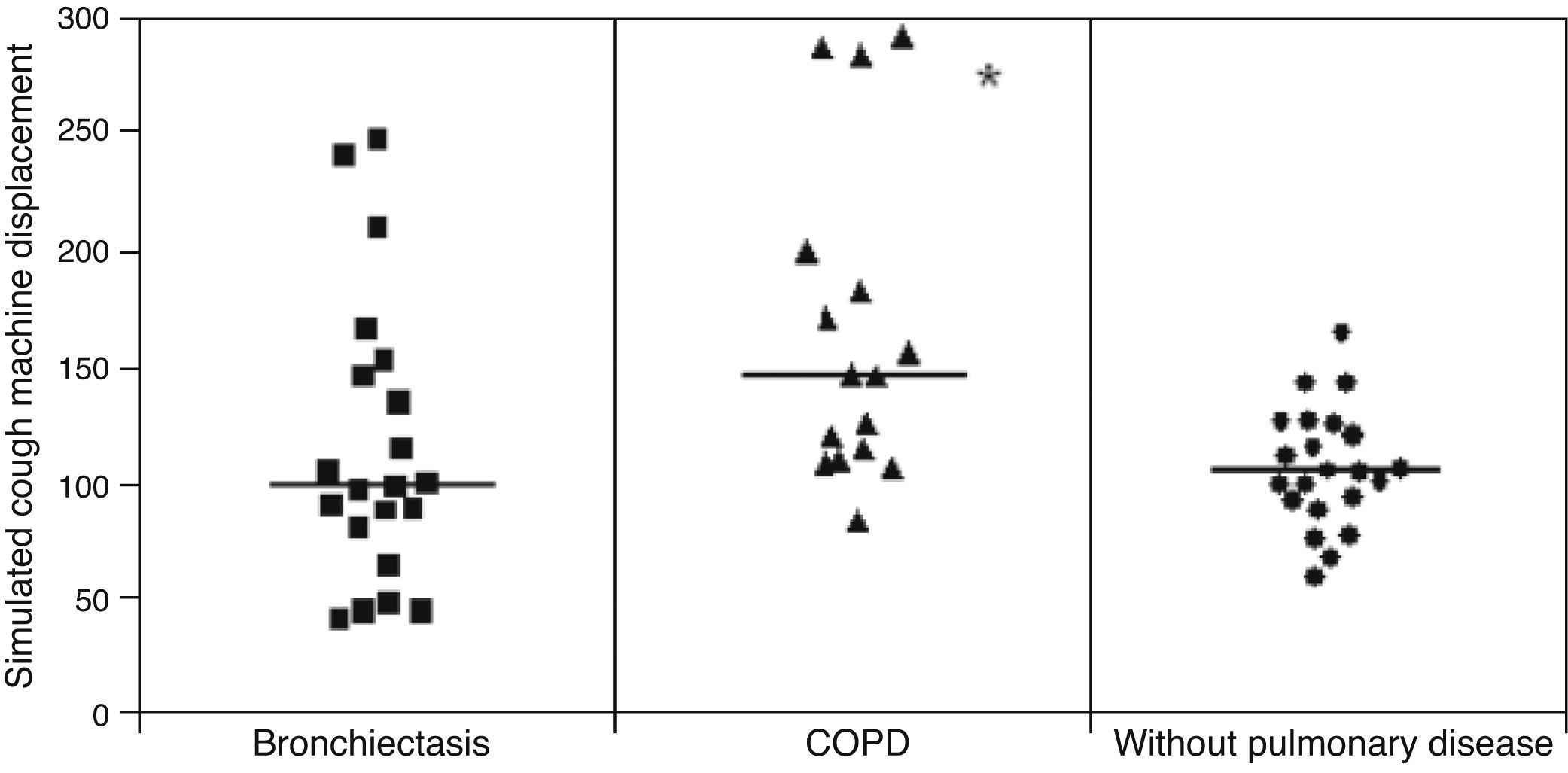
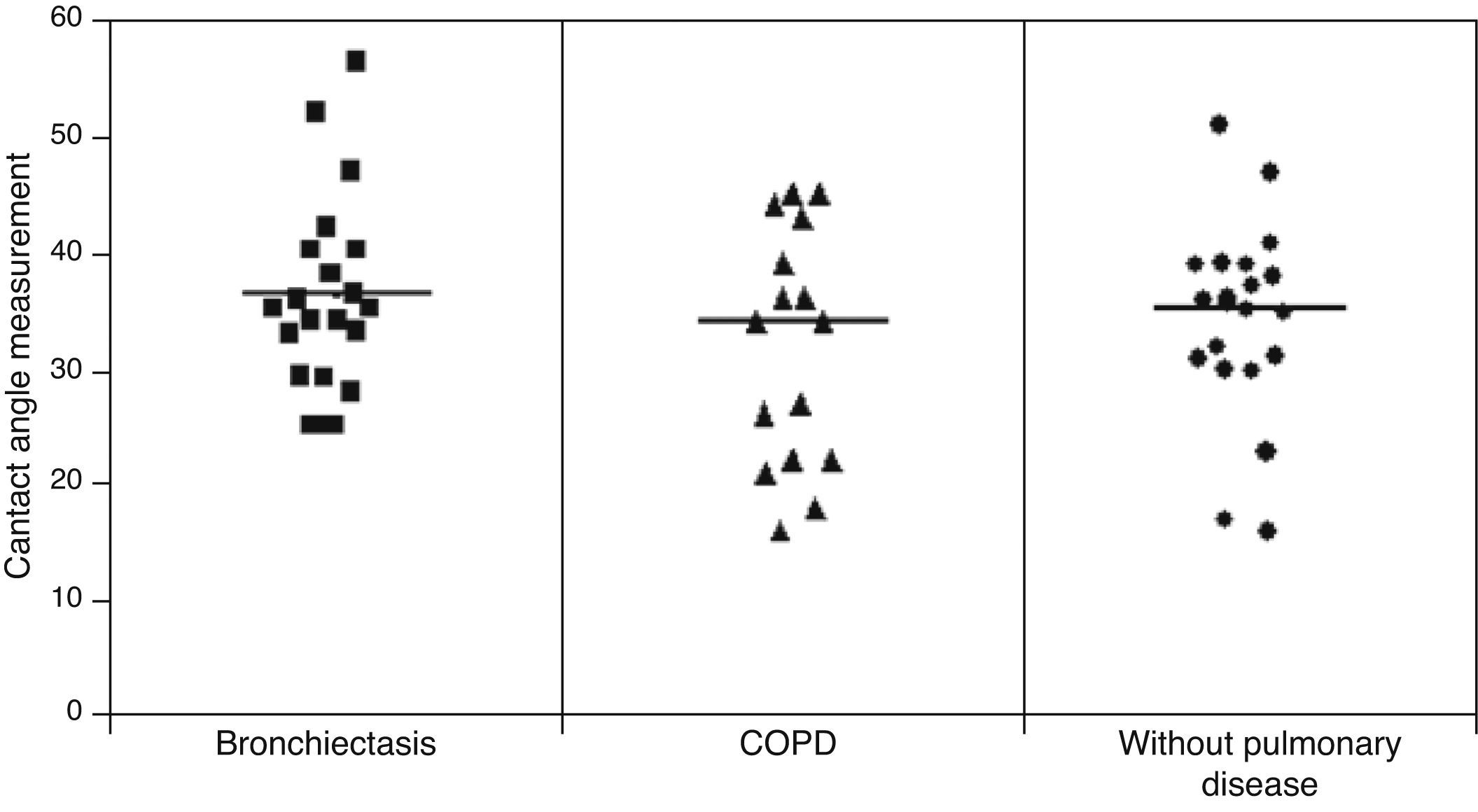
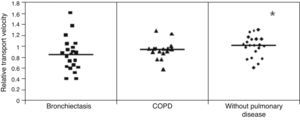
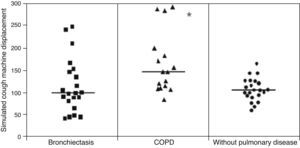
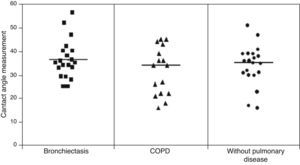
ResultsMCT was found to be greater in healthy individuals (1.0±0.19) than in COPD (0.91±0.17) and bronchiectasis (0.76±0.23) patients (p<0.05), whereas SCM was greater in COPD patients (16.31±7.35cm) than in patients with bronchiectasis (12.16±6.64cm) and healthy individuals (10.50±25.8cm) (p<0.05). No significant differences were observed between the groups regarding CAM.
ConclusionMucus from healthy individuals allows better mucociliary transport compared to that from patients with lung diseases. However, the mucus from COPD patients allows a better transport by coughing, demonstrating that these individuals have adapted to a defence mechanism compared to patients with bronchiectasis, who have impairment in their ciliary and cough transport mechanisms.
Analisar e comparar as propriedades de transporte in vitro da secreção respiratória de aspeto mucoide (M) de indivíduos sem doença respiratória e de pacientes com doença pulmonar obstrutiva crónica (DPOC) e bronquiectasias estáveis.
MétodosForam avaliadas 21 amostras de indivíduos sem doença pulmonar submetidos a processos cirúrgicos, 10 amostras de pacientes com DPOC e 16 amostras de pacientes com bronquiectasias quanto ao transporte mucociliar (TMC), deslocamento na máquina simuladora de tosse (MST) e ângulo de contacto (AC).
ResultadosMaior TMC das amostras de indivíduos sem doença respiratória (1,0±0,19) quando comparado com o dos pacientes com DPOC (0,91±0,17) e bronquiectasias (0,76±0,23) (p<0,05), enquanto que o deslocamento na MST foi maior nos pacientes com DPOC (16,31±7,35cm) quando comparado com o de pacientes com bronquiectasias (12,16±6,64cm) e de indivíduos sem doença respiratória (10,50±25,8cm) (p<0,05). Não houve diferença envolvendo a avaliação do AC.
ConclusãoO muco respiratório dos indivíduos saudáveis tem um melhor transporte ciliar do que o de pacientes com doenças de pulmão. No entanto, o muco de pacientes com DPOC tem uma melhor transportabilidade pela tosse, sugerindo que esses pacientes apresentam adaptações para tais mecanismos de defesa, enquanto que os pacientes com bronquiectasias têm deficiência no transporte ciliar, assim como no transporte pela tosse.
The respiratory system presents several defence mechanisms against inhaled harmful particles from the external environment.1 Mucociliary transport is considered to be the main mechanism in healthy individuals, eliminating the inhaled particles by an effective interaction between mucus and cilia.
Under normal conditions, respiratory mucus spreads out to form a 5-μm layer throughout the bronchial tree, with secretion being minimally produced and varying from 10 to 100ml/day, which is not enough to stimulate the cough receptors.2 However, under adverse conditions such as respiratory diseases (e.g. COPD or bronchiectasis) there is an increase in the thickness of the mucus layer due to alterations in rheological and surface properties and macroscopic aspect, thus favoring the production of more viscous mucus. These alterations impair mucociliary clearance, which could be compensated by the cough mechanism.3–5
Mucus transport in different diseases is related to several factors including the mechanism of the diseases, infection episodes, duration of the disease, aggressiveness of the disease, and drugs used.4–7 since all of them can promote changes in the mucus properties, resulting in different macroscopic aspects and transport by ciliary system or air flow.8,9
Therefore, the objective of the present study was to compare the in vitro transport properties of respiratory mucoid mucus secretion in individuals with no lung disease with stable patients with COPD and bronchiectasis.
Materials and methodsThis study was approved by the local Ethical Committee, and the subjects signed an informed consent form.
Mucoid mucus samples were collected from 47 individuals as follows:
- •
21 healthy volunteers with no lung disease who had undergone surgical intervention, including general anesthesia and orotracheal intubation;
- •
10 stable patients with chronic obstructive pulmonary disease (COPD) who were selected according to criteria established by the American Thoracic Society (ATS);
- •
16 patients with non-cystic fibrosis bronchiectasis confirmed by computerized tomography.
Bronchiectasis and COPD: patients were instructed to cough and expectorate into a glass container covered with gauze so that saliva was separated and sample contamination reduced. Then, the samples were stored in eppendorf tubes filled with vaseline oil to avoid dehydration10 and frozen.11
Healthy volunteers: samples from the group that did not have a respiratory disease were collected from the mucus retained in the endotracheal tube immediately following removal using a cotton stick according to criteria and procedures described by Rubin et al.12
In vitro mucociliary transportThe frog palate is a convenient system for studying mucociliary transport, since the frog's palate epithelium is similar to that of the airways of vertebrates. Anesthetized frogs were decapitated, their jaws disarticulated, and the upper portion of the head was removed. The frog palate was kept in a refrigerator at 4°C for two days covered with plastic wrap in a humidified chamber to deplete the frog mucus. Ciliary activity was maintained under these experimental conditions. The transport of a mucus sample placed upon a mucus-depleted frog palate was determined using a stereomicroscope equipped with a reticulated eyepiece. The velocity of the mucus sample to be tested was compared to the transport speed of autologous frog mucus, and the results are expressed in terms of the relative speed.13–15
Cough transportabilityIn vitro cough experiments employ an apparatus called the “simulated cough machine” adapted from King et al.16 A compressed air cylinder with a pressure gauge serves as a gas supply. Gas release is controlled by a solenoid valve at the outflow port of the cylinder. This is followed by an “upstream resistance”, which serves to make the flow-time profile of the simulated cough comparable to that of human coughing. Mucus transport is calculated by determining the displacement of mucus with the aid of a millimeter ruler.13–15
Contact angle measurementThe contact angle represents the surface tension of the mucus on plane and solid surface. The sample was prepared as described above and the angle formed between mucus and glass surface was measured by using a goniometer at 20× magnification. The glass surface used for these analyses was treated with sulphochromic acid to remove electrical charges. The sample was assessed only once and measured in degree.14,15
Statistical analysisANOVA and then Tukey's test were used for statistical analysis, followed by Spearman correlation. The significance level of 5% (0.05) was adopted for each test.
The sample size was calculated for the cough machine transport as the main outcome, based on data from Tambascio et al.14 considering an average difference for 2.41, a standard deviation of 1.88, a power to 90% and 5% of α. The sample size was estimated for 10 patients.
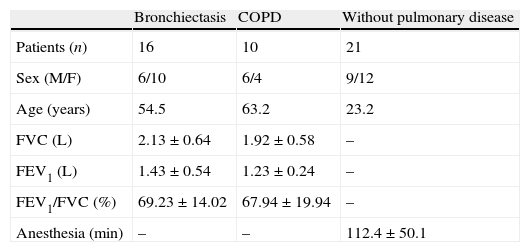
ResultsTable 1 shows data on number of individuals per group, gender, age, spirometric values, and anesthesia duration, covering the 47 subjects, divided into healthy, bronchiectasis, and COPD groups.
Number of patients, sex, age, FVC, FEV1, FEV1/FVC and duration of anesthesia for the groups of bronchiectasis, DPOC and without pulmonary disease.
| Bronchiectasis | COPD | Without pulmonary disease | |
| Patients (n) | 16 | 10 | 21 |
| Sex (M/F) | 6/10 | 6/4 | 9/12 |
| Age (years) | 54.5 | 63.2 | 23.2 |
| FVC (L) | 2.13±0.64 | 1.92±0.58 | – |
| FEV1 (L) | 1.43±0.54 | 1.23±0.24 | – |
| FEV1/FVC (%) | 69.23±14.02 | 67.94±19.94 | – |
| Anesthesia (min) | – | – | 112.4±50.1 |
With regard to the history of smoking, the bronchiectasis group had one smoker (0.7 pack/year on average) and three ex-smokers (5-year smoking cessation on average), whereas the COPD group had two smokers (respectively, 30 and 34 pack/year on average) and seven ex-smokers (8-year smoking cessation on average).
Mucus relative velocity was greater in the healthy individuals than in the COPD and bronchiectasis groups: (p<0.05) healthy group=1.0±0.19, COPD group=0.91±0.17 and bronchiectasis group=0.76±0.23s (Fig. 1).
Displacement of mucus secretion in the simulated cough machine was greater in COPD patients than in the healthy group and bronchiectasis patients: (p<0.05): healthy patients=10.50±25.8cm, COPD=16.31±7.35cm and bronchiectasis 16.31±7.35cm (Fig. 2).
No statistically significant difference was found among the three groups in relation to the contact angle measurement: (healthy=34.16±8.41°; COPD=34.5±10.9°, bronchiectasis=36.78±7.56°) (Fig. 3).
Macroscopically, all samples exhibited a mucoid aspect.
DiscussionIn the present study, we assessed the mucus transport from healthy individuals as well as from patients with chronic obstructive pulmonary disease and bronchiectasis. The results showed that mucus samples from healthy individuals had greater relative mucociliary transport velocity than mucus from lung diseases, whereas COPD patients showed greater mucus displacement in the simulated cough machine compared to healthy and bronchiectasis subjects.
These results confirm that the respiratory mucus from healthy individuals is appropriate for ciliary transport whereas the mucus alterations in COPD patients are more appropriate for cough transport. However, in patients with bronchiectasis both the ciliary and cough mechanisms have been impaired by the intense lung damage.
Other authors have assessed the mucus transport in subjects with bronchiectasis,17–19 chronic bronchitis,17 and cystic fibrosis,17 but the macroscopic aspect or other parameters that would indicate the degree of purulence of the mucus sample were not evaluated. Lopez-Vidriero and Reid20 found a relationship between viscosity and chemical markers of respiratory secretion exhibiting different macroscopic aspects in patients with cystic fibrosis, chronic bronchitis, asthma, and bronchiectasis. However, they did not present any mucoid mucus samples from individuals with bronchiectasis to compare with our results.
As far as we know, this is the first controlled study on both ciliary and cough transportability in patients with COPD and bronchiectasis which only analyzed mucoid secretion in order to reduce the confounding factors involved in the impairment of mucus transport. Regardless of the underlying disease, increased purulence may be related to structural features of the respiratory mucus, thus altering the rheological profile of the mucus secretion and its transport rates.8
Several studies in the literature correlated respiratory secretions with a purulent appearance, which is characterized by its very strong yellow/green color, in patients with bronchiectasis,9,21 COPD,22,23 and chronic bronchitis21 with the presence of inflammatory markers21 and bacteria.9,22,23 Stockley et al.21 suggest that even using only the scales for assessing macroscopic aspect when there are more than two alternatives used, there is a good agreement between evaluators in classifying the purulent or mucoid appearance sample.21
The samples from the patients with lung disease were collected by expectoration, since this is the most practical and efficient way of obtaining non-invasively respiratory mucus from the airways in addition to reflecting the mucus transportability within trachea and bronchi.10 Because of the insufficient amount of mucus produced by healthy individuals, we need to use invasive methods to collect enough amount of secretion and the study of human respiratory mucus is rather difficult to perform.
In our study, we have used a method describe by Rubin et al.12 in which mucus was collected from the endotracheal tubes of patients submitted to minor interventions, then a comparison made of the characteristics of the mucus left on the inner and outer surface of the tube. The authors observed that in both cases the viscoelastic properties were similar, although the mucus left on the inner side of the tube was found to be less hydrated. In our study, where the objective was to use more homogeneous samples, the mucus was collected only from the inner part of the endotracheal tube. The values found for the relative mucociliary transport velocity on frog palate were greater than 0.7, thus corroborating the normal values reported by Puchelle et al.24
In this group, the mean duration of the anesthesia has been 112.4±50.1min and in this group they presented normal values of transport. Besides this a review study carried out by Houtmeyers et al.7 suggests that the relative transport velocity is within the normal range and the rheological properties are unchanged after anesthesia.
The higher mucociliary transportability in the healthy individuals compared to the patients with COPD and bronchiectasis can be explained by the interference of the pathological process itself in these diseases, but age difference between the groups may have played a role as healthy individuals exhibited a considerably lower mean age than that of patients with lung disease.6,17
With regard to the mucus transport by cough, the samples from COPD patients showed greater displacement in the simulated cough machine compared to that of healthy individuals and patients with bronchiectasis. This finding corresponds to the evolution of the pathological processes of the respiratory epithelial damage in the mucus of COPD patients that tends to impair the mucociliary clearance, and would be compensated by the cough mechanism.3 So, the cough mechanism becomes the main transport mechanism as the mucociliary transport is no longer sufficient.25
The mucus from our patients with bronchiectasis showed a slower mucus ciliary transport and there is no easy explanation for this finding. This result is in agreement with results from Daviskas et al.26 and Valente et al.27 and other studies comparing the transportability of respiratory secretions between healthy and patients with bronchiectasis have also found low values for ciliary transport and mucus displacement in the simulated cough machine.27–29 However, it should be emphasized that simulated cough machine systems are not entirely comparable.28,29
Daviskas et al.26 found a greater mean value for contact angle measurement (51.1±2.8°) than that obtained in our study (36.78±7.56°). Puchelle et al.29 demonstrated that high values of contact angle are associated with the impairment of the ciliary transportability and of the cough transportability of bronchial mucus. According to Girod et al.5 a mucus contact angle around 20° provides better protection and lubrification. In our study, very few samples had contact angles which were less than 20° and no significant difference was observed among the groups assessed.
It is thought that some drugs might interfere with mucus transport. In our study, only one patient was taking mucolytics, but a recent review established no clear effect of these drugs on mucus transport.30 This patient exhibited a better ciliary transport despite the decreased cough transport and greater contact angle, a finding which does not correspond to a possible beneficial mucolytic action. Apart from this other drugs used for routine treatment of patients with COPD and bronchiectasis such as bronchodilators, corticosteroids, and antibiotics have no clearly defined effect on the mucus transport.7
LimitationOther confounding factors, such as inflammatory/infection markers, time of disease or number of infection episodes, were not controlled. There was no pattern concerning the collection of mucus whereas the different stages of expectoration.
ConclusionsWe conclude that respiratory mucus of healthy individuals has a better ciliary transport than of patients with lung diseases, while the mucus from patients with COPD has a better cough transportability, suggesting that these patients exhibit adaptations for such defense mechanisms, whereas patients with bronchiectasis have impairment in their ciliary and cough transport mechanisms.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Lima Afonso J, Tambascio J, Dutra de Souza HC, Jardim JR, Baddini Martinez JA, Gastaldi AC, et al. Transporte de secreção mucoide de indivíduos saudáveis e pacientes com doença pulmonar obstrutiva crónica e bronquiectasias. Rev Port Pneumol. 2013;19:211–216.