Delay in diagnosis or treatment of ocular tuberculosis can result in loss of vision. However, due to the fact that early diagnosis is rarely achieved, there are still a broad variety of diagnostic and treatment approaches.
Our aim was to reach a consensus on the management of diagnosis and treatment of ocular tuberculosis.
MethodsCritical appraisal of the literature and expert opinion on diagnosis and treatment of ocular tuberculosis.
Results and conclusionThe currently recommended method for ocular TB diagnosis is screening for tuberculosis in any uveitis of unknown etiology, recurrent or not responding to conventional therapy; in ocular findings highly suggestive of ocular TB and before immunosuppression (particularly biologic agents). TB screening in these cases includes tuberculosis skin testing and interferon gamma testing, along with complete medical history, ophthalmologic evaluation and chest imaging. Positively screened patients should be treated for active tuberculosis with 4 drugs (isoniazid, rifampicin, pyrazinamide and ethambutol) for 6–9 months. Patients should be reviewed at the end of the initiation phase (two months) and at the end of the overall treatment (6–9 months).
Intraocular tuberculosis (IOTB) is a great mimicker of various uveitis entities. This ability to mimic other infections is due in part to the location of infection, the host response, and the virulence of the organism.1 The incidence of tuberculous uveitis (a presumed diagnosis) depends largely upon individual risk factors and the tuberculosis burden of the region.
As most of the patients with tuberculous uveitis do not have other systemic manifestations of the disease, the definitive diagnosis of tuberculosis would require isolation of Mycobacterium tuberculosis through procedures such as vitreous aspiration, aqueous paracentesis or retinal biopsy (with intrinsic risk), which have low sensitivity due to the small volume of material attainable.
Delay in diagnosis or treatment can result in vision loss but since the diagnosis is rarely achieved, there still are a broad variety of approaches to this clinical entity.
In this position paper, delegates from the Tuberculosis Committee of the Portuguese Society of Pulmonology and the Portuguese Society of Ophthalmology, coordinated by the Portuguese Tuberculosis National Program have compiled a critical revision of the literature on diagnosis, treatment and overall management of ocular tuberculosis.
The main aim of this paper is to standardize the procedures used to diagnose and treat ocular tuberculosis. This document summarizes the current knowledge and provides expert consensus recommendations on questions where scientific evidence is still lacking.
MethodologyThe available literature was reviewed and the search for evidence included hand-searching journals, reviewing previous guidelines and searching electronic databases including MEDLINE and PubMed. Final decisions for formulating recommendations were based upon the result of the literature review and the practical experience of the experts.
BackgroundWhat should be the algorithm approach in the diagnosis of uveitis?Complete past medical historyA detailed medical history is the key to diagnosis in the majority of cases of uveitis.2 Medical history should focus on the immune status and the presence of collagen vascular diseases, infectious diseases (including tuberculosis, HIV, syphilis, Lyme and herpetic diseases) and inflammatory bowel disease. Knowledge of underlying diabetes, hypertension or coronary artery disease is essential for optimizing therapeutics.
Review of SystemsThe review of systems is essential for developing a differential diagnosis for uveitis. A uveitis medical questionnaire can be used. Items of particular importance include history of oral or genital ulcers; tinnitus or hearing loss; headaches; malaise; chronic cough; shortness of breath; recent weight loss or gain; fevers, chills, or night sweats; recent contact with individuals with known tubercular disease; diarrhea or blood in the stool; skin rashes; arthritis (axial or peripheral); high-risk sexual activities; ingestion of undercooked meats or tainted water supplies; presence and types of pets; insect bites; or recent foreign travel. Based on the initial interview, the clinician will have established a reasonably complete differential diagnosis before examining the patient. It is important to observe the patient's overall health, noting in particular signs such as pallor and nutritional status.3
Ophthalmic examinationA complete ophthalmic examination should be performed. The conjunctiva should be examined for injection and granulomas. The following anterior segment findings should be documented: the presence of scleritis or keratitis; the presence, distribution, and qualitative characteristics of keratic precipitates; SUN scoring of anterior chamber cell and flare (Table 1); anterior and posterior synechiae; lens opacity or precipitates; and vitreous haze score (standardized Nussenblatt scheme). A complete dilated examination is mandatory, and the following should be recorded by drawing and photography: quality, quantity, and location of vitreous cells; optic nerve edema, hyperemia, pallor, and cupping; cystoid macular edema and choroidal neovascularization; presence, size, quality, and location of retinal and choroidal lesions; and state of the peripheral retina (with scleral indentation), including “snowbanking” (organized inflammatory cells on the inferior pars plana), neovascularization, and chronic retinal detachment. B-scan ultrasonography, optical coherence tomography, and fluorescein angiography are often necessary.3
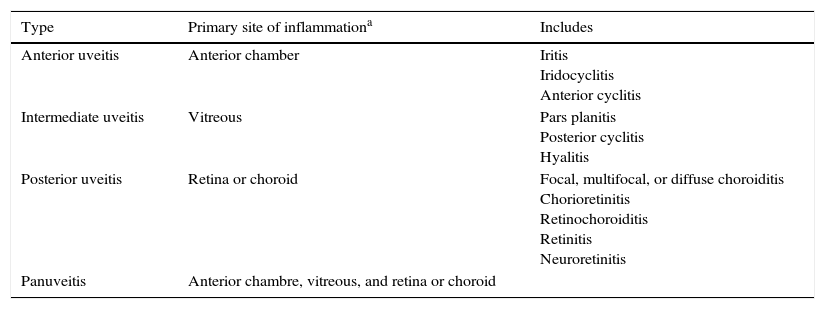
The SUN* Working Group Anatomic Classification of Uveitis.
| Type | Primary site of inflammationa | Includes |
|---|---|---|
| Anterior uveitis | Anterior chamber | Iritis Iridocyclitis Anterior cyclitis |
| Intermediate uveitis | Vitreous | Pars planitis Posterior cyclitis Hyalitis |
| Posterior uveitis | Retina or choroid | Focal, multifocal, or diffuse choroiditis Chorioretinitis Retinochoroiditis Retinitis Neuroretinitis |
| Panuveitis | Anterior chambre, vitreous, and retina or choroid |
As determined clinically. Adapted from the International Uveitis Study Group anatomic classification.4
Classification of uveitis is the first step toward developing a list of potential diagnoses that will help to determine appropriate diagnostic testing, guide therapy, and help determine prognosis.2
In 2005, the world's major uveitis societies initiated a standardization of nomenclature process (Standardization of Uveitis Nomenclature). The group established language for describing the presentation, chronicity, anatomic location, and severity of uveitis and its response to treatment (Table 1). The group affirmed that an anatomic classification of uveitis should be used as a framework for subsequent work on diagnostic criteria for specific uveitic syndromes and that the classification of uveitis entities should be on the basis of the location of the inflammation and not on the presence of structural complications.4
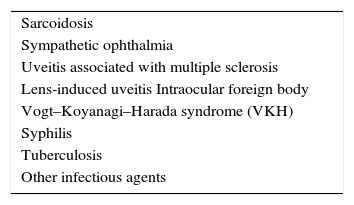
It is very important to classify the uveitis in granulomatous or nongranulomatous. This is made according to the appearance of keratic precipitates (KPs). The more common nongranulomatous type of KP is characterized by fine white collections of lymphocytes, plasma cells, and pigment. These precipitates can form in any disease and cause an anterior uveitis; the finding of nongranulomatous KPs does not help tremendously in the formulation of a differential diagnosis other than to alert the clinician that anterior inflammatory disease has occurred in the eye. Granulomatous KPs are large, greasy-appearing collections of lymphocytes, plasma cells, and giant cells. The finding of granulomatous KPs, also called ‘mutton-fat’ KPs, on slit-lamp examination can be a useful diagnostic clue. Patients with granulomatous KPs usually have a history of a chronic disease with an insidious onset, and frequently have posterior segment disease in addition to their anterior segment inflammation. Other ocular findings suggestive of granulomatous inflammation are iris nodules and choroidal granulomas. Importantly, the finding of granulomatous inflammation in the eye suggests a unique set of diagnostic possibilities that are listed in Table 2.2
Causes of granulomatous inflammation in the eye.2
| Sarcoidosis |
| Sympathetic ophthalmia |
| Uveitis associated with multiple sclerosis |
| Lens-induced uveitis Intraocular foreign body |
| Vogt–Koyanagi–Harada syndrome (VKH) |
| Syphilis |
| Tuberculosis |
| Other infectious agents |
Work-up will not be necessary if:
- •
First episode of a mild anterior acute, unilateral, sudden onset, nongranulomatous uveitis, without symptoms or signs suggesting a systemic disease.
- •
Uveitis in the context of a known systemic disease, for example, a patient with ankylosing spondylitis presenting with an anterior uveitis.
- •
Presence of considered pathognomonic signs of some ocular entity, for example, herpetic keratouveitis (dendritic corneal ulceration, hypertensive uveitis, iris zonal atrophy), Fuchs heterochromic iridocyclitis, etc.
Baseline work-up:
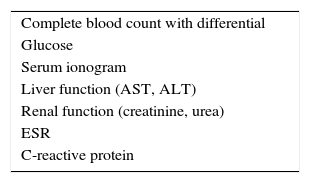
A complete blood count (CBC) with differential can be useful in identifying systemic infection (with leukocytosis), parasitic infection (with eosinophilia), leukemia or certain immunocompromised states. A comprehensive metabolic panel can reveal renal or hepatic dysfunction and undiagnosed hyperglycemia. The results of these tests are also essential for initiating therapy with oral corticosteroids or steroid-sparing medications (Table 3).3Nonspecific tests of inflammation, such as erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP), are sometimes included in the general laboratory evaluation.
There are a number of factors that support the use of laboratory tests to screen for syphilis in most patients with uveitis. Syphilis remains a common cause of uveitis and is easily treatable. Patients with untreated ocular syphilis often have devastating visual outcomes. Importantly, serologic tests for syphilis can be both extremely sensitive and specific.2
Given the prevalence of sarcoidosis as a cause of several uveitis entities, a chest radiograph and serum angiotensin-converting enzyme levels should be included in the baseline work-up. Even in atypical forms of uveitis with a lower likelihood of sarcoidosis it should nevertheless be ruled out since it is treatable and entails a particular prognosis.5–8
Anterior nongranulomatous uveitisApproximately 40–50% of acute anterior uveitis cases are associated with HLA-B27. Acute, unilateral, sudden onset, nongranulomatous, anterior uveitis (particularly with hypopyon) is characteristic of HLA-B27-positive uveitis. Such patients should be questioned carefully for history of axial arthritis, particularly low back stiffness and pain worse on wakening. Although sacroiliac films can be diagnostic of ankylosing spondylitis, their sensitivity is relatively low. A positive review of systems should prompt referral to a rheumatologist for workup.
- •
Baseline work-up;
- •
Syphilis serology;
- •
HLA B27;
- •
Other tests, according to clinical findings.
- •
Baseline work-up;
- •
Syphilis serology;
- •
HLA B27;
- •
Lysozyme in patients on ACE inhibitors;
- •
TB screening;
- •
Other tests, according to clinical findings. For example, in patients with symptoms compatible with multiple sclerosis, an MRI should be ordered.
- •
Baseline work-up;
- •
HLA B27;
- •
TB screening;
- •
Consider serologic testing for Lyme disease, Toxocara, toxoplasmosis and cat scratch disease;
- •
Consider cerebral MRI;
- •
Other tests, according to clinical findings.
Additional tests can be performed according to the clinical findings. An exhausted review of those tests is not the aim of this work.
When to include tuberculosis screening in the diagnostic work up of uveitis?Although any part of the eye can be involved, some signs are more likely to represent intraocular TB.9,10 The most common clinical presentation appears to be posterior uveitis, followed by anterior uveitis, panuveitis, and intermediate uveitis.1,11 According to Gupta and colleagues,12 who are from a TB endemic area, ocular signs consistent with intraocular TB may be assumed in the presence of cells in anterior chamber or vitreous along with:
- 1.
Broad posterior synechiae;
- 2.
Retinal perivasculitis with or without discrete choroiditis/scars;
- 3.
Multifocal serpiginoid choroiditis;
- 4.
Choroidal granuloma (single or multifocal);
- 5.
Optic disk granuloma;
- 6.
Optic neuropathy.
Of all the signs listed above, broad posterior synechiae, retinal vasculitis with or without choroiditis/scars and tuberculous multifocal serpiginoid choroiditis showed high specificity.12
Intraocular TB may present with a wide spectrum of clinical findings,5 many of which are less suggestive than those mentioned above. In non-endemic areas, there were no uveitis features characteristic of TB uveitis and a wide range of manifestations was seen ranging from non-granulomatous anterior uveitis to occlusive retinal vasculitis.13
In non-TB-endemic areas, broad or extensive posterior synechiae occur much more frequently in eyes with HLA-B27 or sarcoid-associated uveitis than intraocular TB and so make this particular sign may be less predictive in such contexts.14 In non-endemic areas, the most common ocular features described were diverse but retinal occlusive vasculitis and serpiginoid choroiditis were common.15,16
Multifocal serpiginoid choroiditis and Eales’ disease (a form or retinal vasculitis) are two entities which are closely associated with a tuberculous etiology.17–20
According to the clinical symptoms and the probability of the disease we propose:
Not to routinely include tuberculosis screening in the following situations:
- 1.
Patients with a known diagnosis (HLA B27 positive, VKH, etc.), unless prolonged immunosuppression or biologic treatment is anticipated;
- 2.
First episode of anterior mild uveitis;
To include tuberculosis screening:
- 1.
Uveitis of unknown etiology, recurrent or not responding to conventional therapy;
- 2.
Ocular findings highly suggestive of ocular TB;
- 3.
Before immunosuppression, particularly before biologic agents.
When tuberculosis diagnosis is considered, the screening process for active tuberculosis requires a combination of a detailed medical history, chest radiology and sputum collection facing respiratory symptoms or abnormal radiology suggestive of pulmonary tuberculosis.
Microbiological study of ocular materialThe definitive diagnosis of tuberculosis would require isolation of M. tuberculosis through procedures such as vitreous aspiration, aqueous paracentesis or retinal biopsy (with intrinsic risk). Samples can be sent for microbiological study (smear, culture and nucleic acid amplification tests (NAAT), which have low sensitivity due to the small volume of material attainable, non-uniform distribution of bacteria in such specimens, presence of NAAT inhibitors and lack of proper gold standard for evaluation of its diagnostic potential.21 Recent studies using multi-targeted PCR have shown better results.21–23 For the diagnosis of presumed tubercular uveitis multi-targeted PCR using three target genes, namely, IS6110, MPB64, and protein b, had a sensitivity of 77.77%, a specificity of 100%, a positive predictive value of 100% and a negative predictive value of 88.88%.22
However, collecting fluid samples from the eye can represent an additional risk in already inflamed eyes, particularly vitreous samples. The selection of patients is crucial. In patients with anterior chamber cells, along with clinical signs suggestive of ocular TB it around 150–200μl of aqueous humor by anterior chamber paracentesis can be collected, under strict aseptic precautions. In selected patients, like those receiving therapeutic vitrectomy for non-resolving intermediate uveitis, vitreous samples can also be used for PCR. Under these circumstances, multi-target PCR has been described as positive in 70.2% of patients.23 In the case of vitrectomy, an epiretinal membrane which has been peeled off during surgery can also be tested for the presence of M. tuberculosis by PCR.24
Patients with positive PCR for M. tuberculosis who were treated with antituberculosis chemotherapy showed resolution of inflammation without recurrence. These results suggest that antituberculosis treatment in PCR positive patients leads to resolution of inflammation and elimination of recurrences, most likely by eliminating M. tuberculosis from the intraocular tissues.25
Immunological testsIf there are no other signs of active tuberculosis, immunological memory tests against M. tuberculosis (tuberculin skin and/or interferon gamma test) should be performed in the same way as screening for latent tuberculosis.26
When to assume an ocular tuberculosis diagnosis?Ocular diagnosis is confirmed following the identification of M. tuberculosis in one of the ocular products collected.
When that is not possible and the patient presents signs suggestive of ocular tuberculosis in association with IGRA or TST positivity (≥10mm in immunocompetent patients and ≥5mm in immunocompromised patients) or chest CT suggestive of untreated TB sequelae, active TB should be assumed and patients should start an anti-tuberculosis regimen.27
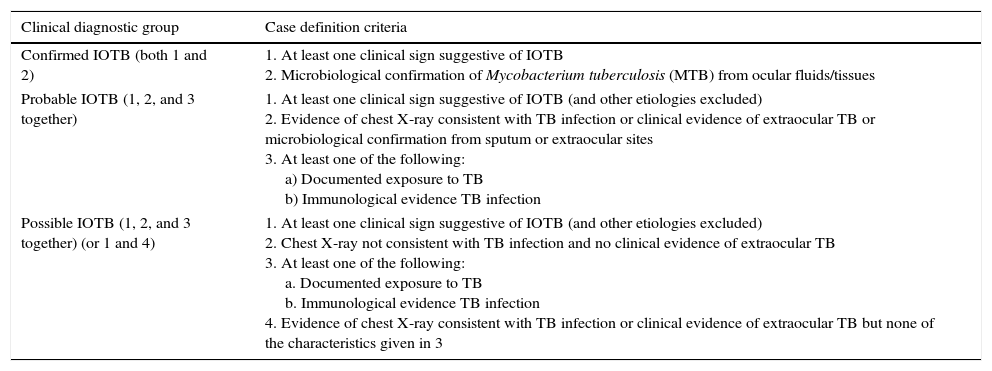
Gupta proposed a classification of intraocular tuberculosis divided in “Confirmed IOTB”, Probable IOTB” and “Possible IOTB” (Table 4).12
Classification of intraocular tuberculosis12.
| Clinical diagnostic group | Case definition criteria |
|---|---|
| Confirmed IOTB (both 1 and 2) | 1. At least one clinical sign suggestive of IOTB 2. Microbiological confirmation of Mycobacterium tuberculosis (MTB) from ocular fluids/tissues |
| Probable IOTB (1, 2, and 3 together) | 1. At least one clinical sign suggestive of IOTB (and other etiologies excluded) 2. Evidence of chest X-ray consistent with TB infection or clinical evidence of extraocular TB or microbiological confirmation from sputum or extraocular sites 3. At least one of the following: a) Documented exposure to TB b) Immunological evidence TB infection |
| Possible IOTB (1, 2, and 3 together) (or 1 and 4) | 1. At least one clinical sign suggestive of IOTB (and other etiologies excluded) 2. Chest X-ray not consistent with TB infection and no clinical evidence of extraocular TB 3. At least one of the following: a. Documented exposure to TB b. Immunological evidence TB infection 4. Evidence of chest X-ray consistent with TB infection or clinical evidence of extraocular TB but none of the characteristics given in 3 |
A 4-drug anti-tuberculosis treatment (isoniazid, rifampicin, ethambutol, and pyrazinamide) is proposed over a period of 6–9 months. Factors affecting the treatment outcome in patients with presumed ocular tuberculosis on anti-tubercular therapy (ATT) were analyzed in a retrospective review (the largest on this subject, involving 175 patients) at a tertiary referral eye care center in a low endemic area. Failure was defined as recurrence of inflammation within 6 months of completion of ATT. It was found that patients with intermediate uveitis or panuveitis and those on immunosuppressive therapy had greater tendency for treatment failure while those with more than 9 months of ATT had a lower likelihood of failure.28 A decision to start treatment implies that it is maintained for a period of at least six months, even if there seems to be no clinical response after two months.19
From the moment a decision to treat is made, treatment should not be stopped because of lack of response, unless other diagnosis has been made.29 The lack of response can be due to an immune reaction that needs anti-inflammatory treatment.
There seems to be no benefit in TB prophylaxis in regard to uveitis control.30
How to access the effectiveness of ocular TB's treatment?Treatment efficacy versus failure has been difficult to define. Recent studies31 have proposed that failure be defined as inability to taper oral corticosteroids to less than 10mg/day or topical steroids to less than twice/day, inability to stop oral immunosuppressive agents as well as persistence/recurrence of inflammation within the first 6 months of completion of tuberculosis treatment.
Patients should be reviewed at the end of the initiation phase (two months) and at the end of treatment (6–9 months).13 The revision should include:
- 1.
Obvious TB lesions (e.g. conjunctival or choroidal granuloma), looking for indications of lesion reduction with progressive atrophy and pigment mobilization.10
- 2.
Any uveitis compatible with TB (positive TST or IGRA tests) but no obvious ocular TB lesion and no evidence of systemic disease, signs of reduction in inflammatory response and/or ease of titration of anti-inflammatory drugs (e.g. corticosteroids):32,33
- a.
patients with idiopathic uveitis and a positive IGRA test have been shown to react favorably to antituberculosis treatment with improvement in ocular inflammation, even though only a small minority had documented (prior) tuberculosis or a history of exposure14;
- b.
previous work has shown resolution of inflammation after TB treatment in about 70% of patients, a change in the nature of their inflammation in 18% and no benefit in 11%13;
- c.
in the example of serpiginous-like choroiditis, antitubercular therapy significantly reduced recurrences. Lesions responded to combined antitubercular and steroid therapy, usually spared the fovea and had good final visual acuity.18
- a.
- 3.
In any case, bear in mind:
- d.
response to TB treatment may be masked by concomitant anti-inflammatory treatment1;
- e.
the natural course of this ocular disease may be indolent or characterized by an alternating exacerbation-remission course.
- d.
In addition to the aforementioned and mandatory reviews at two months after initiation of ATT and at the end of treatment, patients should also be assessed every two to three months for:
- 1.
Maintenance of inflammatory control–remission;
- 2.
Absence of corticosteroid dependence;
- 3.
Reduction in disease flare-up or recurrence rate (lower severity, longer disease-free periods).18
Follow-up is otherwise identical to uveitis entities unrelated to TB, being based essentially on disease severity.34 Such involvement may be assessed in the following terms:
- 1.
Is there any threat to central vision?
- 2.
Is the disease progressing?
- 3.
What is the patient's immune status (immunocompetent/immunosuppressed)?
- 4.
Is there the possibility of a masquerade syndrome (e.g. other infectious disease or neoplasm)?
Drug-associated toxic optic neuropathy is a rare but vision-threatening condition. Diagnosis is made based on an extensive case history and careful clinical examination. The examination findings include varying decrease in vision, normal pupillary reflexes and extraocular muscle function, and unremarkable fundoscopy, although swollen optic discs may be found in the acute stage of the optic neuropathy. Other important findings suggestive of toxic optic neuropathy include decreased color vision and cecocentral visual field defects. It is therefore of paramount importance to identify any contributing factor for optic nerve toxicity so that they may be properly addressed in any patient with worsening or non improving signs or symptoms of visual disfunction.35
Prospective studies of ocular toxicity in patients receiving ethambutol have been conducted as a part of directly observed treatment strategy. Ethambutol has been shown to cause ocular toxicity at standard doses (>15mg/kg/day), making early recognition of ocular symptoms a priority to prevent unnecessary delay in diagnosis and irreversible visual loss.36
The incidence and prognostic factors of ethambutol-related optic neuropathy (EON) have been addressed in retrospective studies. One such study found a prevalence of 1.29% (62 out of 1004 patients) with visual impairment and subsequent diagnosis of EON. Sixteen patients (26%) were being followed for over 6 months when symptoms developed. Of these, eight patients (50%) showed visual improvement (an increase in visual acuity of ≥2 Snellen lines) after ethambutol was discontinued. The other eight patients (50%) showed no visual improvement. When analyzing multiple factors between the patients with and without visual improvement (body weight, daily dose of ethambutol, duration of ethambutol use, cumulative dose of ethambutol, renal function, underlying disease such as diabetes mellitus or hypertension, and initial visual acuity) there was no statistically significant difference and as such no obvious prognostic factors were found to facilitate visual recovery.31
It is advisable to obtain informed consent – toxicity may occur with any treatment dose despite regular ophthalmic follow-up, and subsequent visual loss may be severe, bilateral and irreversible.
- 1.
Perform baseline (pre-treatment) ophthalmic examination including assessment of visual acuity, confrontation visual field testing, color vision and dilated fundus observation.
- 2.
Perform optic coherence tomography (OCT) because of a higher likelihood of early toxicity detection (especially if normal baseline examination). Decreased retinal ganglion-cell layer (RGL) thickness and volume suggest optic nerve toxicity. RGL analysis may thus contribute to early diagnosis and management of EON.37,38
- 3.
Contrast sensitivity testing, pattern visual evoked potentials (VEP) and multifocal electroretinography (ERG) are sensitive tests to detect ethambutol toxicity in subclinical stages and hence useful for monitoring patients under ethambutol therapy for ocular toxicity. These may be considered complementary to the baseline examination, should they be available, particularly in dubious cases.39,40
- 4.
Ethambutol should be discontinued as soon as EON is suspected, and as soon as visual symptoms develop (visual acuity loss, color vision disturbance, scotomata). The patient should be referred promptly to ophthalmic evaluation.31
- 5.
Frequency of ophthalmic screenings should be every two months for standard ethambutol doses (equal or higher than 15mg/kg/day), or monthly if additional risk factors for toxicity are present such as:
- a.
diabetes mellitus
- b.
chronic renal disease
- c.
alcoholism
- d.
elderly patients
- e.
children
- f.
other ocular diseases (other than the uveitis)
- g.
ethambutol-induced peripheral neuropathy
- h.
linezolid-related optic neuropathy35,41
- 6.
Systematic close monitoring (probably monthly) with VEP is advisable in children younger than 5 years old since ethambutol-related visual impairment has also been shown in this age group, and such alterations in visual function are most likely underestimated.42
When another etiology for the uveitis has been identified, and the patient has immunological tests such as tuberculin skin or IGRA test positive or evidence of untreated past tuberculosis and being considered for immunosuppressive therapy.43,44
Recommendations- 1.
Patients with uveitis of unknown etiology, recurrent or not responding to conventional therapy or ocular findings highly suggestive of ocular tuberculosis should always be screened for tuberculosis.
Grade of recommendation: C
- 2.
Patients with a known diagnosis (HLA B27 positive, VKH, etc.) or with a first episode of anterior mild uveitis, unless prolonged immunosuppression or biologic treatment is anticipated, do not need to be screened for tuberculosis.
Grade of recommendation: C
- 3.
Whenever possible, samples should be collected for micobacteriological study (smear, culture, NAAT and drug susceptibility test).
Grade of recommendation: C
- 4.
Tuberculosis screening should always include a symptoms questionnaire, chest radiography, tuberculin skin testing and/or IGRA test.
Grade of recommendation: C
- 5.
In the presence of a confirmed TB diagnosis or in its absence a positive immunological diagnosis in the presence of suggestive ocular disease, patients should be started on a full treatment regimen with four drugs (isoniazid, rifampicin, pyrazinamide and ethambutol), for an overall estimated period of 6–9 months.
Grade of recommendation: C
- 6.
Patients should be assessed every 2/3 months for improvement in inflammatory signs, absence of steroid dependence and reduction in recurrence rate.
Grade of recommendation: C, D
- 7.
Adverse events should be monitored, particularly ethambutol toxicity. We propose baseline ophthalmic examination (visual acuity, confrontation visual field testing, color vision and dilated fundus observation) along with OCT. If available, contrast sensitivity testing and electrophysiology testing (pattern VEP and/or multifocal ERG) may be considered complementary to the baseline examination as they improve the diagnostic yield, and thus can be ordered at the physician's discretion. Screening is to be repeated every two months or monthly should there be risk factors. Ethambutol is to be discontinued as soon as optic neuropathy is suspected (visual symptoms or abnormalities in the aforementioned tests).
Grade of recommendation: C
The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.