Influenza affects millions of people worldwide each year and can lead to severe complications, hospitalizations, and even death, especially among vulnerable populations such as older adults and those with chronic medical conditions. Annual vaccination is considered the most effective measure for preventing influenza and its complications. Despite the widespread availability of influenza vaccines, however, vaccination coverage rates remain suboptimal in several countries. Based on the latest scientific evidence and expert opinions on influenza vaccination in older people and patients with chronic disease, the Portuguese Society of Pulmonology (SPP), the Portuguese Society of Diabetology (SPD), the Portuguese Society of Cardiology (SPC), the Portuguese Society of Geriatrics and Gerontology (SPGG), the Study Group of Geriatrics of the Portuguese Society of Internal Medicine (NEGERMI-SPMI), and the Portuguese Society of Infectious Diseases and Clinical Microbiology (SPDIMC) discussed best practices for promoting vaccination uptake and coverage and drew up several recommendations to mitigate the impact of influenza. These recommendations focus on the efficacy and safety of available vaccines; the impact of influenza vaccination on older adults; patients with chronic medical conditions, namely cardiac and respiratory conditions, diabetes, and immunosuppressive diseases; and health care professionals, optimal vaccination timing, and strategies to increase vaccination uptake and coverage. The resulting position paper highlights the critical role that vaccinations play in promoting public health, raising awareness, and encouraging more people to get vaccinated.
Influenza, commonly known as the flu, is a highly contagious respiratory disease caused by influenza viruses.1 It affects millions of people worldwide each year and can lead to serious complications, hospitalizations, and even death, especially among vulnerable populations, such as older adults, children under 5 years old, pregnant women, and people with chronic medical conditions.1–3 In Portugal, the influenza season typically starts in October and peaks in January or February, causing a significant health and economic burden.4
Annual influenza vaccination is considered the most effective measure for preventing both influenza and its complications.1,2 It is recommended for everyone over 6 months of age, especially those at high risk of severe illness or complications (older adults, young children, pregnant women, and people with underlying medical conditions, such as diabetes, cardiovascular disease (CVD), and chronic obstructive pulmonary disease [COPD]).1,2
Although influenza vaccines are widely available, vaccination coverage rates remain suboptimal in several countries. Portugal has very good coverage rates among older people, but uptake needs to be improved in certain groups, such as people aged 60–65 years, people with comorbidities, pregnant women, and health care professionals. Advances in vaccine technology have also improved the efficacy of current vaccines.5
The Portuguese Society of Pulmonology (SPP), the Portuguese Society of Diabetology (SPD), the Portuguese Society of Cardiology (SPC), the Portuguese Society of Geriatrics and Gerontology (SPGG), the Study Group of Geriatrics of the Portuguese Society of Internal Medicine (NEGERMI-SPMI) and the Portuguese Society of Infectious Diseases and Clinical Microbiology (SPDIMC) came together to discuss and debate best practices for promoting vaccination coverage and reducing the burden of influenza. The resulting position paper discusses different aspects of influenza vaccination in older people and people with chronic conditions and proposes recommendations grounded in the latest scientific evidence and expert opinion.
MethodsIn February 2023, a narrative non-systematic literature search of MEDLINE/Pubmed database was conducted, between 2013 and 2023, using the following search on papers’ title: (influenza OR flu) AND (vaccination OR vaccine) AND (("pulmonary diseases" OR (“chronic obstructive pulmonary disease” OR COPD) OR pneumonia) OR (("cardiovascular diseases" OR CVD) OR “myocardial infarction”) OR diabetes OR (elderly OR "older adults" OR geriatrics) OR (hospitalizations OR strategies OR burden OR epidemiology) OR (immunodepressed OR immunosuppressed OR immunocompromised)). Only publications with full text available and in English, Portuguese or Spanish were considered. The authors also proposed other publications throughout the preparation of the present document, which were added to the final references, namely international guidelines and recommendations.
The expert panel, composed of two pulmonologists, one cardiologist, two endocrinologists, one infectious disease specialist, and three geriatric specialists, analyzed and selected the articles of each area for their relevance to set a position on influenza vaccination, with a focus on recent publications. The members of the panel were selected and invited by each of the Scientific Societies based on their knowledge and expertise in this field.
What is the impact of influenza in Portugal and the world?Influenza infection is characterized by annual seasonal epidemics and sporadic, unpredictable global pandemics. It remains one of the leading causes of morbidity and mortality worldwide. While the true burden of influenza is probably underestimated, World Health Organization estimates about 3 to 5 million cases worldwide of severe illness annually, with a particularly high incidence among vulnerable populations.1 CDC reports over 35 million cases in the US in the 2019–20 season2 and, in Europe, before the COVID-19 pandemic, influenza was the infectious disease with highest burden in terms of annual incidence-based disability-adjusted life years (DAILYs), with a mean incidence of nearly 10%,6 which corresponds to more than 1 million annual cases in Portugal.
The Burden of Acute Respiratory Infections (BARI) study, a real-world evidence study that analyzed the burden of influenza in Portugal between 2008 and 2018, estimated a mean annual hospitalization rate for influenza of 11.6 cases per 100,000 population. The corresponding estimated rate for influenza-associated deaths across all age groups was 22.7 cases per 100,000 population, rising to 102.5 per 100,000 in people over 65 years old.4 Even though this study demonstrated the burden of influenza in Portugal, its findings also pointed to a significant underdetection of cases. Influenza-associated deaths occur annually, but not all cases are coded as such on death certificates, which translates to excess mortality and underreporting.4
The gold standard laboratory tests for influenza are reverse transcription PCR, a quick, highly sensitive and specific molecular test, and viral culture, which is essential for the characterization of novel viruses, surveillance of sensitivity to antiviral drugs, and monitoring of antigenic drift.7
Most patients recover from influenza within a few days, but complications, including pneumonia and death, can occur, especially in high-risk populations such as patients with underlying immune deficiencies.7,8 The World Health Organization (WHO) recommends yearly immunization for the most vulnerable population groups.1 According to the Vacinómetro initiative, which has been monitoring influenza vaccination coverage rates among risk groups in Portugal since the 2009–2010 season, coverage rates have exceeded the recommended target of 75% in people aged ≥65 years since 2019–20, contrasting with the situation in most other European Union (EU) countries.9
How does flu impact hospitalizations?In the United States, according to the Centers for Disease Control and Prevention (CDC), an estimated 29 million influenza infections occurred in the 2018–19 season, with 380,000 cases needing hospitalization.2 These figures can increase significantly in high-severity seasons, such as the 2017–18 season with 41 million confirmed cases and 710,000 associated hospitalizations.2 In 2017–18 and 2018–19, most patients hospitalized for influenza were >65 years old (66% and 55%, respectively).2
The Portuguese BARI study detected a mean of 11.6 influenza-associated hospitalizations per 100,000 population a year; 46% of the patients were >65 years old (26.5 cases per 100,000 population). Overall, 65.6% of admissions corresponded to patients with comorbidities, and 62% of these were aged >65 years. The incidence of influenza-associated excess respiratory or cardiovascular hospitalization was estimated at a mean annual incidence of 51.5 cases per 100,000 population across all age groups and 199.6 cases per 100,000 population in the over-65 group. It should be noted that hospitalizations in the private sector (nearly 23% of total hospitalizations in 2018) were not analyzed.4
The BARI findings also point to an underestimation of the clinical and economic burden of influenza. Over the 10-year period analyzed, direct influenza hospitalizations cost the Portuguese national health system (NHS) €41.2 million. This corresponds to a direct annual cost of €3.9 million for influenza-associated hospitalizations, 78.6% of which were among patients with comorbidities.4 These direct annual costs, whilst certainly underestimated, are considerable and highlight the importance of investing in free vaccination programs, especially for more susceptible groups.
In conclusion, influenza represents a significant clinical and economic burden both nationally and internationally, and patients with comorbidities and those aged >65 years appear to be most at risk.
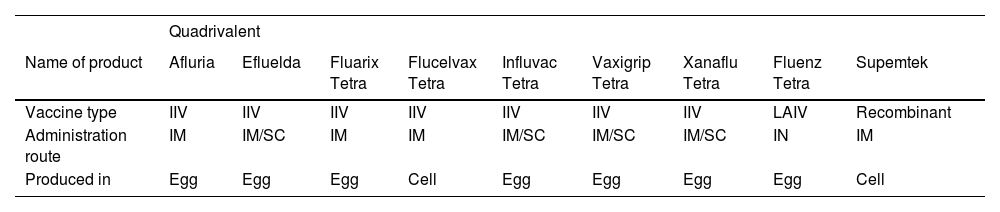
What flu vaccines are available and how effective are they?Vaccination is an effective and affordable way to control influenza viruses and ultimately safeguard public health.10 Vaccines can be classified by their valence (number of strains).5,11 Trivalent vaccines have three strains: two influenza A strains and one influenza B strain. Quadrivalent vaccines containing two influenza A and two influenza B strains have been developed and approved to provide additional protection against circulating influenza B viruses from a second lineage.5,11 Currently available quadrivalent vaccines are summarized in Table 1.
Seasonal quadrivalent influenza vaccines available in the European Union and the United States of America (2019–2020 season) according to the European Centre for Disease Prevention and Control (ECDC) (Adapted from ECDC, 2019146).
IIV, influenza-inactivated vaccine; IM, intramuscular; IN, intranasal; LAIV, live attenuated Influenza vaccine; SC, subcutaneous.
Vaccine development is informed by predictions about which strains will be in circulation the following season. These strains are selected by a panel comprising experts from over 100 national influenza centers, including the WHO, following a review of surveillance and laboratory data. In February, the expert panel reviews data for the Southern Hemisphere and selects strains for the next influenza season in the Northern Hemisphere.11
Portugal recommends annual immunization with influenza-inactivated vaccines (IIV)s. Live attenuated influenza vaccines (LAIVs) also exist, but not in Portugal until 2023. For the 1999–2000 influenza season, IIVs were estimated to have a respective efficacy of 77% and 91% against influenza A (H3N2) and influenza A (H1N1) virus infection among children aged 1–15 years.12 Another study reported an efficacy of 66% in children aged 6–24 months.13 Vaccine efficacy rates in the range of 16% to 75% have been reported by several randomized controlled trials (RCTs) in adults aged <65 years.14–16 Data from an aggregate data meta-analysis showed a vaccine effectiveness rate of 52% in adults aged >60 years for seasons when the vaccines and circulating strains were antigenically well matched and 36% when they were mismatched.17
The effectiveness of LAIVs can also vary from year to year according to age and risk groups and virus types and subtypes.18–20 Vulnerable populations—pregnant women, immunocompromised patients, and people aged >50 years or <2 years21—are not eligible for these vaccines due to their replicative capacity and the increased risk of adverse events.
According to the Portuguese NHS, three vaccines, all quadrivalent IIVs, were available for the 2022–23 season: Influvac Tetra, Vaxigrip Tetra, and Efluelda (the last one only for nursing home residents).
Are flu vaccines safe?Vaccines are recommended for children and younger and older adults, and their safety is crucial to maintaining trust and increasing adherence to national vaccination programs. Like all medicines, however, the possibility of adverse events (including rare events) cannot be completely ruled out, even though all vaccines meet rigorous safety standards and are strictly reviewed before authorization.22
Influenza vaccines are safe and well tolerated by children.23 Nevertheless, IIVs may be more likely to cause injection-site pain, redness, and swelling than LAIVs, which, in addition, are contraindicated in immunosuppressed individuals and close contacts, pregnant women, children under two and adults over 50 years old.23
Although some studies have reported a modest increase in the risk of febrile seizures in young infants after immunization in combination with other vaccines,24,25 most studies have not found any association.26–28
Several studies have shown that vaccines have a robust safety profile and acceptable reactogenicity in adults and older people. In both populations, injection-site pain is the most common local adverse effect, while fatigue, headache, and myalgia are the most common systemic effects.29
It is uncertain whether influenza vaccination increases risk for Guillain-Barré syndrome (GBS). A case-control study conducted between October 2010 and May 2011 in seven Italian regions identified a small but significant association between influenza vaccination and GBS, a rare neurological autoimmune disorder.30 Another study linking universal health care databases from Ontario, Canada, with data gathered between 1993 and 2011 identified approximately 3000 hospital admissions for GBS; 330 of the patients had received an influenza vaccine and 109 had an influenza-coded health care encounter in the 42 weeks prior to hospitalization. The risk of GBS was lower for vaccination than for influenza-coded health care encounters (1.03 GBS admissions per million vaccinations vs 17.2 GBS admissions per million influenza-coded health care encounters), supporting the theory that influenza vaccination may reduce the small risk for GBS that can be triggered by influenza virus infection.31
In another study involving more than 3 million persons/ year, between 1995 and 2006, from the Kaiser Permanente Northern California (KPNC), there was no evidence of an increased risk of GBS after any vaccination, including influenza.32
The concerns about the risk of inducing GBS in mass immunization programs against H1N1 2009 do not seem justified by the available epidemiological data; however, the experiences from the 1976 swine flu vaccination program emphasizes the importance for active and passive surveillance of vaccine safety monitoring.33
Accordingly, in the present time, a second flu shot is not recommended in individuals who have previously developed GBS within 6 weeks of an initial shot. If exposed to influenza, these patients could be treated with antiviral chemoprophylaxis.34
Allergic reactions to egg have also been reported in association with influenza vaccination. Most influenza vaccines are produced using egg-based technologies and therefore contain a small quantity of egg protein, such as ovalbumin. Nonetheless, findings from studies evaluating injectable and nasal spray vaccines in people with and without egg allergies suggest that severe reactions in those with allergies are rare.35–40 Health organizations recommend that people with a history of severe allergic reactions to egg should be vaccinated in an inpatient or outpatient setting under the supervision of a health care provider able to recognize and manage severe allergic conditions.41 Alternatives to egg-based vaccines for those with severe allergies include cell culture–grown and recombinant vaccines.
Vaccines, like all medicines, can have adverse effects, but these are generally mild and disappear within a few days. Overall, influenza vaccines are safe and well tolerated and remain the single best defense against influenza and its complications. Influenza is the main preventive adult infectious disease and vaccination represents the most effective and safest measure to prevent flu and its complications. The flu vaccine is useful and beneficial to all people aged six months or more with a permanent safety record. When universal vaccination is not possible, equitable criteria should be established to protect patients at risk of developing serious forms of the disease or complications.
COPD is the third leading cause of death worldwide. In 2019, it caused 3.23 million deaths across the globe.42 Acute exacerbations are the main cause of COPD-associated morbidity and mortality. The most common cause of exacerbations is tracheobronchial infection,43 with respiratory viruses involved in approximately 30% to 50% of cases.44–49 COPD is the most prevalent underlying disease among hospitalized patients with acute respiratory illness during an influenza epidemic, indicating that it is one of the main risk factors for a worse outcome.50–52
Vaccination is the cornerstone of efforts to mitigate the effects of influenza. Most national vaccination technical advisory organizations recommend both pneumococcal and annual influenza virus immunization in patients with pulmonary diseases and chronic lung illness.53–55 Numerous large series have shown that immunization of older patients with COPD against influenza reduces hospitalizations, deaths, and health care costs.56–58 Wang et al.59 analyzed mortality data for 35,637 vaccinated people aged >65 years over a period of 10 months in southern Taiwan and found that vaccination strongly reduced major cause–specific mortality, even in patients with COPD (hazard ratio, 0.55; 95% CI, 0.41–0.73).
Specific studies have analyzed the link between vaccination and COPD exacerbations. One meta-analysis of 11 RCTs, some involving patients with COPD, indicated that vaccines, particularly inactivated ones, significantly reduced the number of exacerbations per patient compared with placebo.60,61
Two UK studies showed that influenza vaccination was safe for COPD patients and did not increase exacerbations.62,63 Similar findings were observed for other respiratory illnesses, such as asthma.62
Immunization may also have advantages over no vaccination in terms of preventing negative clinical outcomes, including death, in patients with COPD, with some studies showing that influenza vaccination significantly lowers all-cause mortality, respiratory event–related fatalities, and acute coronary syndrome events in this setting.59,64
In summary, decreased lung function following influenza infection and increased susceptibility to additional infections from other agents can trigger COPD exacerbations. Prevention is crucial.65
What effect does influenza have on hospitalizations for community-acquired pneumonia?Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality worldwide.66 In the United States, it accounts annually for nearly 4.5 million outpatient and emergency room admissions and 24.8 admissions per 100,000 individuals, with a greater incidence among older patients.67,68 In the Portugal NHS, CAP was responsible for 3.7% of all-cause hospitalizations among adults in general and 7.0% among those aged ≥65 years between 2000 and 2009.69
The influenza virus is one of the main causative pathogens in CAP.66 A South Korean study evaluating the prevalence and clinical features of pneumonia in 3253 patients with laboratory-confirmed pH1N1 virus infection concluded that at least 5% of those infected with the virus will develop CAP.70 Other studies of hospitalized patients with pneumonia in the United States and China have also reported significant influenza infection rates.67,71
The influenza virus is also linked to increased rates of CAP-associated hospitalizations and deaths. A multivariate analysis comparing clinical manifestations of H1N1 influenza A CAP and non-influenza CAP found higher mortality and intensive care unit (ICU) admission rates in patients with influenza.72 These worse outcomes can be explained by the ability of the virus to dysregulate both innate and adaptive immune responses, leading to secondary bacterial infections, in particular those caused by the gram-positive species Staphylococcus aureus and Streptococcus pneumoniae.73 In a prospective, observational study conducted from 2009 to 2015 in a large cohort of ICUs in Spain, 16.6% of almost 3000 patients diagnosed with influenza had a coinfection.74 The risk factors identified were age and immunosuppression. Influenza clearly increases the likelihood of hospitalization for CAP, and testing patients presenting to the emergency room, especially during peak influenza activity, ensures appropriate treatment and in-hospital infection control.75 Here, once again, vaccination might be the most viable option. An observational, multicenter study investigating the effect of prior influenza vaccination on disease severity and mortality in patients with CAP showed that immunization was associated with less severe clinical presentations and greater long-term survival.76
A Danish study of 34,000 citizens aged 65–79 years in 2021–22 showed that recipients of a high-dose quadrivalent influenza vaccine had lower hospitalization rates for influenza or pneumonia than those who received the standard dose (0.2% vs. 0.4%). All-cause mortality rates were also lower (0.3% vs. 0.7%). There were no significant differences in serious adverse events between the high-dose and standard-dose groups.77
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) also recommends pneumococcal vaccination for all adults with COPD due to high rates of coinfection, in particular by the S. pneumoniae.54 The most common cause of COPD exacerbations is tracheobronchial infection, with respiratory viruses involved in approximately 30% to 50% of cases. The immunization of older patients with COPD against influenza reduces hospitalizations, deaths, and health care costs. The influenza virus is one of the main causative pathogens in CAP and it is also linked to increased rates of CAP-associated hospitalizations and deaths. The influenza vaccination is associated with less severe CAP clinical presentations and greater long-term survival.
CVD has been linked to influenza infections since the 1930s, when an increase in cardiovascular deaths was observed during influenza seasons.78 It has since been hypothesized that infection with the influenza virus can induce a thrombophilia state and thrombosis of a pre-existing, subcritical atherosclerotic plaque, resulting in acute coronary occlusion and ultimately acute myocardial infarction (AMI).78 It can also cause tachycardia and hypoxia and trigger the release of inflammatory cytokines, potentially contributing to AMI through multiple mechanisms.78 Influenza respiratory infections and AMI have a temporal link, which is strongest in the first 3 days but can persist for up to 1 year.79 Subsequent studies demonstrated a 6- to 10-fold increased risk of AMI in the first week of influenza infection and a 3- to 8-fold increased risk of stroke. In a large study of almost 2 million hospital admissions for AMI, patients with concomitant influenza infection (1%) were found to have worse outcomes than those with isolated AMI.80 In short, influenza-related respiratory infections not only increase the risk of AMI, but also affect the prognosis of hospitalized patients, resulting in additional complications, longer average hospital stays, and higher mortality.65
Findings from both observational studies and RCTs suggest that influenza vaccination may help reduce cardiovascular events, particularly in high-risk groups.81 Observational studies have shown that influenza vaccination decreases the risk of AMI by 19% to 45%. This protective effect is similar to that reported for other primary cardiovascular prevention strategies, such as smoking cessation (32%−43%), anti-hypertensive treatment (17%−25%), and statin therapy (19%−30%).78,79,82 More recent data showed a significant reduction in the incidence rate ratio of AMI (0.84, 95% CI 0.78 – 0.91) in a 4-week window after influenza vaccination.83 There is also evidence that older people with a history of AMI have a lower risk of cardiovascular events when vaccinated against influenza viruses.84
Does influenza prevention decrease cardiovascular risk in other CVDs?CVD follows a similar seasonal pattern to influenza. Time-series analyses have demonstrated an increase in CVD risk during or immediately following an influenza epidemic.84 One study of influenza-related hospital admissions found that almost 12% of patients experienced a major cardiac event during hospitalization.85 The same concluded that 31% of patients with influenza and an acute cardiovascular event required critical care and 7% died.85 In this study, the researchers found that patients admitted with acute coronary syndrome who were randomly assigned to receive influenza vaccination experienced fewer major cardiovascular events than controls discharged without immunization.85 Moreover, a meta‐analysis of four RCTs and 12 observational studies, including a total of 237,058 patients, showed that influenza vaccination was associated with a 25% relative risk reduction (RRR) in all‐cause mortality and an 18% RRR in cardiovascular mortality in patients with CVD, probably linked to the 13% RRR observed for major adverse cardiovascular events.86 In a meta-analysis of six RCTs, where patients were randomized to influenza vaccination or placebo, Behrouzi et al.87 found that only 3.6% of those in the vaccination group developed a major adverse cardiovascular event compared with 5.4% in the placebo group; this corresponds to an absolute risk reduction of 1.8% to prevent one event. The results were even better in a subgroup analysis of patients with recent acute coronary syndrome, where only 6.5% of vaccinated patients had one cardiovascular event vs. 11% of patients in the placebo group (absolute risk reduction, 4.5%). Finally, influenza vaccination reduced cardiovascular mortality risk by almost 50% (2.6% vs. 5.4%).87
Despite the benefits demonstrated, influenza vaccination has been historically underutilized in both the general adult population and patients with CVD. In a study of patients with atherosclerotic cardiovascular disease, just 37% of those aged 18–49 years and 55 % of those aged 50–64 years received an influenza vaccination in 2019–2020.88 Annual influenza vaccination (a low-cost, well-tolerated, and easy-to-implement intervention) should be administered, together with other guideline-recommended therapies aimed at reducing cardiovascular risk, to patients with a cardiovascular indication.87,88 This practice is recommended by international clinical practice guidelines,89 particularly for patients with more prevalent and high-risk CVDs, such as heart failure and coronary artery disease.90 The Portuguese Directorate-General for Health (Direção-Geral da Saúde, DGS) strongly recommends vaccination for patients with CVD in its latest guidelines on seasonal influenza vaccination (Standard 006/2023: Seasonal Vaccination Campaign against Influenza: Fall/Winter 2023–2024).91 Influenza infection increases the risk of myocardial infarction and decompensation of chronic heart diseases, such as heart failure. Influenza vaccination decreases the risk of myocardial infarction by 19% to 45%, a magnitude similar to other cardiovascular disease primary prevention strategies. It also reduces the risk of adverse events in patients with acute myocardial infarction.
Diabetes is a global public health problem whose prevalence has more than doubled in the past 20 years; in 2021, 537 million adults (20–79 years) across the world had diabetes.92 According to the National Diabetes Observatory, the prevalence of diabetes in Portugal in the same year was 14.1% in adults aged 20–79 years and 27.1% in those aged 60–79 years.93
Comorbidities such as heart disease are common in people with diabetes. A person with diabetes who develops influenza thus is more likely to develop severe and even fatal complications.94 People with diabetes have higher rates of hospitalization, ICU admission, and pneumonia and influenza-related deaths than those without. The odds of being admitted to an ICU for influenza are three times higher for people with diabetes (odds ratio, 4.29) compared with those without.95
Several case reports and series have reported that influenza virus infection can exacerbate diabetic ketoacidosis, a common presentation of type 1 diabetes.96,97 Moreover, there is research suggesting that chronic hyperglycemia may be the primary cause of influenza complications in persons with diabetes.65 The recruitment of immune cells, neutrophil degranulation, complement activation, and immune cell phagocytosis can all be decreased by hyperglycemia, which can collectively limit the immune response to influenza virus infection.98
Blood glucose fluctuations may have an even greater impact on physiological dysfunction than hyperglycemia. These fluctuations are typically greater and more frequent in people with diabetes compared with healthy people.99 Elevated glucose levels can increase influenza virus replication in pulmonary epithelial cells, cause structural changes to the lungs that reduce pulmonary function, and trigger complications, such as heart and kidney disease.65 Conversely, influenza might also cause fluctuations in glucose levels. One retrospective cohort study determined that influenza was associated with a 75% increase in the frequency of abnormal glucose levels among type 2 diabetes and negatively impacted quality of life.100 In summary, decompensated diabetes increases the risk of influenza complications, and influenza can lead to decompensated diabetes.
Many national immunization technical advisory groups believe that those living with diabetes and other metabolic disorders are high-priority groups for influenza immunization. In Portugal, people with diabetes have been targeted since 2001, and in 2017–18, the NHS introduced free vaccination for this population.101
Many studies support the benefit of seasonal influenza vaccination in people with diabetes. Most of the studies identified in a systematic review on the immunogenicity, safety, and effectiveness of seasonal influenza vaccination in people with diabetes reported comparable immunogenicity in people with diabetes and healthy or non-diabetic participants.102 The seroconversion and seroprotection rates 1 month after vaccination were generally above the recommended criteria for licensure (>40% and >70%, respectively). A retrospective cohort study comprising around 120,000 people with type 2 diabetes found that vaccine recipients had a 24% lower all-cause mortality rate during the influenza season than non-vaccinated participants.103 The above systematic review also found that most studies analyzed reported a significant association between seasonal influenza vaccination and a reduced risk of hospitalization in persons with diabetes. The vaccines were also found to be well tolerated by children with type 1 diabetes and adults with type 1 and 2 diabetes, confirming the safety profile of seasonal influenza vaccination. Another study found seasonal influenza vaccination to be associated with a considerably lower risk of all-cause mortality in people with diabetes, especially those aged >65 years.65
The CDC recommends that persons with diabetes, like those with CVD, should be immunized with IIVs not LAIVs.104
Influenza vaccination of this risk group has been shown to be effective and is even globally advised.1 The American Diabetes Association 2023 Standards of Medical Care in Diabetes caution against using LAIVs in patients with chronic conditions such as diabetes and recommend instead inactive or recombinant vaccines. The standards also suggest an additional benefit from the high-dose quadrivalent inactivated influenza vaccine in individuals aged ≥65 years.105 People with diabetes have higher rates of hospitalization, ICU admission, and pneumonia and influenza-related deaths. Influenza infection can contribute to diabetic decompensation and chronic hyperglycemia may be the primary cause of influenza complications in persons with diabetes. Influenza vaccination of persons with diabetes has been shown to be effective, safe, and associated with lower all-cause mortality and hospitalizations.
Data suggest that patients with seasonal or pandemic influenza have significantly higher morbidity and mortality in the presence of secondary infections or bacterial coinfection.74,106,107 It has been shown that up to 75% of influenza patients who develop pneumonia test positive for bacterial coinfection.108 Acute respiratory failure in immunosuppressed patients admitted to the ICU has been linked to a very high mortality risk,109 although the exact rates and impact of infection in this population are not well understood.110
Nevertheless, the link between delayed antiviral treatment and worse outcomes is clear. Solid organ transplant recipients on immunosuppressive medications are highly susceptible to more severe symptoms when they have influenza.111 Pneumonitis is common in this population and can be fatal in up to 7% of patients.112,113 Acute rejection and graft dysfunction following influenza have been reported in lung and non-lung transplant recipients.114 In renal transplant recipients, H1N1 influenza has been linked to increased allograft dysfunction.112,113
People with HIV are also considered to be a high-risk group for influenza complications. Impaired T-cell reactivity in this population can lead to severe and prolonged influenza infections.115 Influenza-associated hospitalizations and deaths were considerably higher among patients with HIV/AIDS before the widespread use of highly active antiretroviral therapy (HAART). Cardiopulmonary hospitalization rates in this population fell by 53% with the advent of HAART, although they are still higher than in the general population.110
Over 50,000 patients receive hematopoietic stem cell transplants every year worldwide.116 Intensive pretransplantation conditioning, consisting of high doses of chemotherapy and/or radiotherapy, leaves patients severely immunocompromised for months after transplantation. While influenza is relatively uncommon in this setting, it is associated with an increased risk of pneumonia and mortality (up to 43% in the latter case).110,111
Influenza is also a serious complication in patients with hematologic malignancies on chemotherapy, many of whom develop pneumonia.117
Is there an optimal time to vaccinate patients undergoing immunosuppressive therapy?Most international and national organizations recommend vaccination for patients with immunosuppressive conditions.118–121 Vaccines can protect against secondary bacterial infections and influenza. The CDC recommends annual vaccination with IIVs or quadrivalent recombinant vaccines and advises against LAIVs for both immunocompromised people and close contacts.118
The Infectious Disease Society of America recommends annual IIV immunization for immunocompromised individuals older than 6 months.119 This group of patients includes people with HIV infection with solid organ or hematological malignancies and people on long-term immunosuppressive therapy. The recommendation is supported by the CDC, the American Society of Blood and Marrow Transplantation, and the European Group for Blood and Marrow Transplantation, although the recommended windows for vaccination differ. While the US guidelines support lifelong immunization,121 European guidelines encourage tailoring decisions to individual patients.120 Patients who are highly unlikely to respond, such as those receiving intensive induction or consolidation chemotherapy for acute leukemia or those who have recently received anti-B-cell antibodies are the only patients who should not be administered IIVs.119
There is no question that immunization against influenza is essential. It should be noted however, that immunocompromised patients have an increased likelihood of influenza infection and more severe illness and that vaccines are less effective in this population. Additionally, very little information is available on the outcomes of ongoing annual vaccination programs. More clinical trials using pertinent clinical outcome metrics would significantly improve the capacity to recommend vaccinations for immunocompromised patients in a more informed manner.
How important is the prevention of influenza and its complications in healthy ageing?Ageing is an undeniable risk factor contributing to influenza-associated morbidity and mortality. Beyond intrinsic factors such as immunosenescence and physiological changes, chronic disorders further increase older individuals' susceptibility to influenza.122 Yet, environmental factors also play a role, exposing older persons to higher transmission risks in enclosed settings such as health care facilities and senior housing.123 Infection rates, in such cases can be as high as 40%,124 influenced not only by environmental conditions but also by the heightened vulnerability of older residents.
The CDC's definition of influenza-like illness includes fever with a cough or sore throat, but this characterization poses challenges when applied to older populations.18 Although influenza holds a substantial risk of morbidity and mortality among older persons, clinical presentation is often unconventional, with less fever.125 This attenuated fever response might be due to distinct thermoregulatory or baseline temperature shifts. Use of a lower fever threshold for suspected influenza in older adults thus might improve detection.126
Older adults can also exhibit less typical symptoms often overlooked in diagnosis, such as behavioral changes, acute confusion/delirium, perceptual disturbances, anorexia, dehydration dizziness, falls, and incontinence.127 Cognitive impairment further complicates influenza detection as older patients may struggle to communicate symptoms to health care professionals, whether the illness manifests through traditional or atypical symptoms or as exacerbations of underlying chronic conditions, notably heart and pulmonary ailments.126 These challenges contribute to the high rates of underdiagnosed cases, obscuring the recognition of the impact of influenza on older adults and explaining the surprisingly low rates of confirmed influenza in this population.128
Hospitalization rates and length of stay are substantially increased in individuals aged >65 years.129,130 In Portugal, influenza hospitalization rates in this age group are over triple those observed in younger groups.131 Influenza escalates mortality rates, with 90% of related deaths occurring in older individuals.128 It also leads to deterioration of physical, cognitive, and functional abilities, and impaired activities of daily living.132 All these potential repercussions highlight the need for better prevention and management.
According to the WHO, influenza vaccination offers 70% to 90% protection in healthy adults.1 It benefits community-dwelling, institutionalized, and hospitalized older persons. For community-dwelling adults aged ≥65 years, influenza vaccination has been linked to a 48% reduction in mortality risk and a 27% reduction in hospitalization risk.133 Vaccinated hospitalized older patients have lower ICU admission, invasive ventilation, and bacterial pneumonia rates.134 Influenza vaccination is cost effective and can reduce costs by 2.75% and years of life lost by over 50%.134
The WHO thus considers influenza vaccination to be the main preventive strategy in older persons, despite diminished immune responses, and views it as a critical component of healthy aging.1
Future studies should prioritize the creation of an effective, strain-independent influenza vaccine that optimally stimulates the aged immune system.135 Recommending the most effective vaccines remains key. Misbeliefs that vaccination can trigger influenza or cause more severe illness, together with the notion that reduced social contact minimizes transmission, contribute to vaccination reluctance.
Individual, group-based, and community-level health literacy interventions, improved accessibility, and free vaccination could all enhance vaccination uptake and adherence.
Is high-dose influenza vaccine effective in advanced age?Influenza vaccine responses tend to become less effective with age and immunosenescence.136–138 Strategies such as higher antigenic doses and the use of adjuvants have been evaluated as a means of enhancing vaccine immunogenicity and clinical effectiveness in older adults.135 In an RCT comparing a more immunogenic high-dose trivalent vaccine with a standard-dose vaccine during the 2013–14 influenza season, Gravenstein et al.139 observed reduced respiratory-related hospital admissions in nursing home residents aged >65 years who received the high-dose vaccine (3.4% vs. 3.9% over 6 months). In a follow-up study, the authors noted lower mortality (17.1% vs. 18.3%) with the high-dose vaccine and comparable functional decline rates.140 Their findings are corroborated by other studies.141–143 Diaz Granados et al.141 found that high-dose IIVs lowered the risk of pneumonia, cardiorespiratory illnesses, and hospitalization in older adults, benefits that were echoed in a meta-analysis by Lee et al.143 A systematic review comparing the effectiveness of adjuvanted vaccines versus non-adjuvanted standard and high-dose vaccines in adults aged >65 years during multiple influenza seasons showed that the adjuvanted and high-dose non-adjuvanted influenza vaccines were effective alternatives to standard-dose vaccines for older individuals.144 Overall, high-dose influenza vaccines have consistently outperformed standard-dose vaccines across various trials and methodologies, leading the Portuguese NHS to recommend Efluelda, a quadrivalent high-dose vaccine, for nursing home residents. Notably, the benefits also extend to relatively younger community-dwelling older adults.141 Scientific evidence supports the efficacy of high-dose vaccines irrespective of age, comorbidities, frailty, and prior vaccination history.145 As immunosenescence affects vaccine efficacy beyond institutionalized individuals, limiting high-dose vaccines to this population raises ethical concerns. Immunosenescence, physiological changes, and chronic illnesses increase the susceptibility of older people to flu. Flu in older patients is associated with higher hospitalization, length of stay, and mortality. Around 90% of flu-related deaths occur in older people. Flu vaccination is the main prevention strategy for older persons and a critical component of healthy aging Responses to the flu vaccine tend to be less effective in older persons. Strategies such as higher antigenic doses and the use of adjuvants have been shown to increase vaccine immunogenicity and clinical efficacy in older adults.
The most crucial and widespread influenza vaccination goal in Europe is to protect vulnerable populations both directly (by vaccinating older adults and patients with chronic medical conditions) and indirectly (by vaccinating close contacts of people at risk, such as health care professionals and formal and informal health care providers). Since December 2009, the EU Council has urged EU Member States to develop and implement action plans and policies aimed at achieving a seasonal influenza vaccine coverage rate of 75% among people aged ≥65 years.146 Coverage rates have improved across risk groups in Portugal, which achieved a rate of 76% among individuals aged >65 years in 2019–20, reflecting the effectiveness of the country's policies to support and facilitate access to vaccination.9
According to the Portuguese Vacinómetro study, this success is built on three main pillars, the first being easy access to free vaccination. The lowest vaccination coverage rate (42.8%) was observed in people aged 60–64 years.9 Although vaccination is advised in this age group, people aged 60–64 years were not classified as a risk group until 2020, meaning they were ineligible for free vaccination. Older adults, by contrast, have had access to free vaccines since 2012.147,148 These differences in coverage rates highlight the value of offering free influenza immunization to risk groups.9 Numerous studies have shown the benefits of free vaccination policies.149–152 Moreover, vaccination at community health centers, without the need for prescriptions or appointments, as well as pharmacy vaccination programs, are key to increasing influenza vaccination uptake.
The second pillar for success identified was a physician recommendation, particularly when this came from an attending physician or family doctor. According to the Vacinómetro study, a physician recommendation was the main reason that led to influenza vaccination, with both vaccinated and unvaccinated individuals agreeing that a physician recommendation strongly influenced their decision. This was especially striking among pregnant women, where 84.6% of those vaccinated mentioned a physician recommendation as the main reason.9
The third and final pillar for success is increased vaccine literacy and awareness linked to national campaigns led by government agencies and scientific groups. The Vacinómetro initiative revealed that people continue to have misconceptions regarding the potential complications of influenza, despite the significant link between influenza and the development/exacerbation of certain chronic diseases.9,151 This may, however, have changed somewhat with increased awareness stemming from the mass vaccination campaigns during the COVID-19 pandemic.
According to the latest data from the Vacinómetro, just 52.6% of health care professionals in contact with patients have been vaccinated against seasonal influenza since the start of the 2022–23 season, highlighting the need to increase coverage among health care professionals. Vaccination of health care workers protects both workers and their families, reduces absenteeism, and helps control infections in health care environments, with a positive impact on patient transmission and morbidity and mortality.153 It also sets an example for the broader population. Targeted awareness campaigns and onsite vaccination programs could increase influenza vaccination uptake among health care workers.
In summary, widespread access to free vaccines, patient-provider communication, and information are key to a successful vaccination strategy. Widespread access to free vaccines, patient-provider communication, and information represent three main strategies to increase influenza vaccination rates.
These evidence-based recommendations for health care professionals and the general public are designed to emphasize the importance of vaccination in vulnerable populations and address common misconceptions surrounding vaccine safety and effectiveness. The aim of this position paper is to provide a comprehensive understanding of current best practices for influenza vaccination and highlight the critical role that vaccination plays in promoting public health, increasing awareness, and encouraging more people to get vaccinated.
Considering the scientific evidence presented, the SPP, SPD, SPC, SPGG, NEGERMI-SPMI, and SPDIMC present the following recommendations and conclusions:
- •
Influenza vaccination is the cornerstone of efforts to reduce the burden of influenza and its complications, especially in high-risk groups such as older people, young children, pregnant women, and patients with chronic medical conditions.
- •
IIVs are safe and effective, and high-dose inactivated vaccines should be prioritized in all adults aged ≥65 years.
- •
Descriptive studies have shown that influenza vaccination significantly reduces hospitalizations and mortality in immunocompromised patients and patients with respiratory diseases (namely, COPD), CVD, and diabetes.
- •
These high-risk groups should be vaccinated annually, and health care professionals should ensure timely prescription, including at the time of hospital discharge.
- •
Vaccination of health care professionals against influenza is vital given their higher exposure to both the virus (risk of infection) and high-risk patients (risk of transmission). It confers multiple benefits, including infection control in health care environments, lower absenteeism, reduced patient transmission, reduced morbidity and mortality, and promotion of broader vaccination by way of example
- •
The EU Council's target of a vaccine coverage rate of 75% for individuals aged >65 years has been achieved in Portugal thanks to free-of-charge, easily accessible vaccination, physician recommendations, epidemiologic surveillance, and national awareness campaigns.
- •
To further increase vaccination coverage rates, strategies such as health literacy interventions, enhanced accessibility, and no-cost immunizations should be employed.