Induced sputum with hypertonic saline has been suggested as a safer and cheaper alternative to bronchoalveolar lavage for evaluation of patients with interstitial lung diseases (ILD).
ObjectiveTo evaluate the safety and feasibility of sputum induction in ILD and to compare sputum cellular profiles with paired bronchoalveolar lavage fluid results.
Materials and methodsTwenty patients underwent sputum induction with 4.5% saline within 2 weeks of bronchoalveolar lavage. Total, differential cell counts and cellular viability were assessed. Wilcoxon test and Spearman's rank correlation coefficient were used and a p<0.05 was considered statistically significant.
ResultsFrom a total of 20 subjects (mean age 49.4±16.4 years, 70% male) a satisfactory sputum sample was obtained in 15 subjects (75%). Induction was stopped in one subject, due to a significant decrease in PEF. The cell profiles for induced sputum and bronchoalveolar lavage fluid (BALF) were different (p<0.05), except for eosinophils, and there were no significant correlations between the two methods. Compared to sputum reference values there was an increase of lymphocytes (3.2% vs 0.5%) and eosinophils (1.4% vs 0.0%). Comparing sarcoidosis and hypersensitivity pneumonitis sputum, both diseases had an increase in lymphocytes (4.4 vs 3.9%), with a significant higher neutrophil count in hypersensitivity pneumonitis (65.4% vs 10.6% p<0.05), a finding also seen in BALF.
ConclusionInduced sputum is feasible and safe in interstitial lung diseases. Although sputum cellular counts are not correlated with bronchoalveolar lavage fluid, sputum cellular profiles may help to distinguish different ILD.
A indução de esputo com soro hipertónico tem sido apontada como uma alternativa, mais económica e segura, ao lavado broncoalveolar na avaliação de doentes com doença pulmonar intersticial (DPI).
ObjetivoAvaliar a segurança e a exequibilidade do esputo induzido nas DPI e comparar os perfis celulares do estudo com os resultados obtidos por lavado broncoalveolar.
Material e métodosVinte doentes realizaram indução de esputo com soro hipertónico (4,5%) num intervalo de 2 semanas após a realização de lavado broncoalveolar. Foram analisadas as contagens diferenciais e a viabilidade celular. Para a análise foram utilizados os testes de Wilcoxon e a correlação de Spearman's e um valor de p<0,05 foi considerado estatisticamente significativo.
ResultadosDos 20 sujeitos estudados (média de idade 49,4±16,4 anos, 70% do sexo masculino), foi obtida uma amostra satisfatória de esputo em 15 (75%). A indução foi interrompida num doente, devido a uma queda significativa do PEF. Os perfis celulares obtidos do esputo induzido e do lavado broncoalveolar foram distintos (p<0,05), com exceção dos eosinófilos, e não se verificaram correlações estatisticamente significativas entre os 2 métodos. Comparando os resultados do esputo com os valores de referência, verificou-se um aumento de linfócitos (3,2 vs. 0,5%) e eosinófilos (1,4 vs. 0,0%). Quando se compararam os resultados do esputo dos doentes com sarcoidose e pneumonite de hipersensibilidade, ambos os grupos apresentaram um aumento de linfócitos (4,4 vs. 3,9%) e a contagem de neutrófilos estava significativamente aumentada na pneumonite de hipersensibilidade (65,4 vs. 10,6%; p<0,05), achado também presente no lavado.
ConclusãoO esputo induzido é um método seguro e exequível nas DPI. Embora as contagens celulares obtidas não se correlacionem com as do lavado, os perfis celulares do esputo podem ajudar na distinção das diferentes DPI.
Interstitial lung diseases (ILDs), also known as diffuse parenchymal lung diseases, are a heterogeneous group of pulmonary disorders with different etiopathogenesis, treatment and prognoses. Although some reports suggest that the prevalence and incidence of ILDs has increased during recent decades, no population-based longitudinal studies have documented recent trends in ILD subgroups.1After evaluation of symptoms, pulmonary function tests and thoracic imaging, direct invasive methods, such as bronchoalveolar lavage (BAL), transbronchial biopsy and/or surgical lung biopsy are usually required to confirm the diagnosis.2 BAL is the standard procedure recommended in the European Respiratory Society and American Thoracic Society statements for the diagnostic work-up of patients with ILD.2 BAL is useful in the diagnosis of less frequent ILD disorders (e.g. occupational, eosinophilic lung diseases, Langerhans cell Histyocitosis), and to support the diagnosis of the most prevalent ILDs (e.g. sarcoidosis, hypersensitivity pneumonitis, idiopathic pulmonary fibrosis) in the absence of a pulmonary biopsy. In fact, depending on the inflammatory cell pattern revealed in BAL and according to the patient clinical history and radiological findings, the biopsy with inherent risks may be obviated.3 Although BAL is a minimally invasive technique, with minimal risk to patients when administered properly, patient compliance to the procedure is a common problem. Additionally, BAL is not recommended for screening programs, nor to evaluate exposures and follow-up, and is contraindicated in some patients.3–6
Induced sputum (IS) by inhalation of hypertonic saline solution has been suggested as an alternative in some circumstances. It is a safe, non-invasive and reproducible procedure to collect cells and soluble mediators from the airways.7 During the past decade IS has been recognized as a very useful sampling method for both research and clinical use, adding valuable information for the diagnosis, monitoring and treatment response prediction, particularly in chronic obstructive lung diseases such as asthma or COPD.8–11 There have been a few descriptions about IS use in the diagnosis and monitoring of ILDs.3,5,12,13 However, its safety and usefulness in this context remains uncertain and controversial, especially its potential in sampling the immunological and inflammatory events in the deep lung.
In this study, our main aim was to investigate the safety and feasibility of the IS procedure and to describe sputum analysis, when compared with expected reference values, in patients with ILD. In a selected group with lymphocytic alveolitis, we also compared the IS cellular characteristics with those obtained in BALF samples.
Materials and methodsSubjectsTwenty consecutive patients attending the ILD hospital outpatient clinic underwent sputum induction. The procedure was explained to the patients who were encouraged to cough up any sputum after inhalation of 4.5% saline solution for 5min. If no sputum was obtained and lung function was greater than 80% of the baseline, the test continued until a predefined maximum induction time of 45min. Bronchoscopy and BALF processing was performed within 2 weeks from induced sputum examination, as part of routine clinical management, according to the recommended guidelines and previous reports.14,15 The final diagnosis was established by a respiratory physician, based on the BALF results and the clinical, radiological, functional and pathological data. Informed written consent was obtained and the study approved by the Faculty of Medicine of Porto ethical committee.
Sputum induction and processingSputum induction was performed using an inhalation of 4.5% saline through a mouthpiece connected to an ultrasonic nebulizer OMRON NE-U17 (Omron Healthcare Europe, Netherlands) on the maximum output settings. Sputum induction and processing were performed according to the ERS task force recommendations16,17 using hypertonic saline for periods of 5min. Baseline and post 200μg of salbutamol Peak Expiratory Flow (PEF) were registered using Mini-Wright Peak-Flow Meter (Clement-Clarke International, Harlow, Essex, UK). The induction stopped either when the patient produced an adequate sample of sputum or 45min of inhalation were completed or if PEF dropped below 80% of the baseline value. After induction, all sputum macroscopically free of salivary contamination was selected and treated with dithiothreitol (DTT, Sputolysin™ 10% concentration; Calbiochem Corporation, San Diego, CA, USA) in phosphate-buffered saline. The suspension was centrifuged and the cell pellet resuspended and stained with Trypan-blue for viability and total cell numbers per milligram of processed sputum. Coded cytospins were prepared and stained using May–Grünwald Giemsa for differential cell counts of intact bronchial epithelial cells and leukocyte, up to a total of 500 nonsquamous cells. Sputum samples were considered adequate if they contained <80% squamous epithelial cell from saliva.
Bronchoalveolar lavage fluid processingBAL was performed using a fibrotic bronchoscope at the time of diagnosis, according to ERS guidelines.15 In brief, 200ml sterile saline was instilled into the middle lobe subsegment in 50ml aliquots. Each aliquot was immediately and gently aspirated with a syringe. BALF total volume was measured and filtered through sterile double layer surgical gauze to remove mucus and pooled. The first aliquot was excluded. A Neubauer Cell Chamber was used for BALF total cell count and differential cell count on cytospin slides stained with May–Grünwald-Giemsa.
Statistical analysisData are expressed as medians (P25–75). Since variables were not normally distributed, the nonparametric Wilcoxon test was used to compare the differences in differential cell counts and cell subsets. Correlations between different cells from different samples (induced sputum and BALF) were examined by Spearman's rank correlation coefficient. A p value of <0.05 was considered as statistically significant.
ResultsFrom the 20 subjects (mean age 49.4±16.4 years, 70% male) with ILD, 7 had sarcoidosis (35%), 8 (40%) hypersensitivity pneumonitis (HP), and one each (5%) idiopathic pulmonary fibrosis, Cryptogenic Organizing Pneumonia, Respiratory Bronchiolitis associated with Interstitial Lung Diseases, Churg-Strauss syndrome and Silicosis.
A sputum sample satisfactory for processing was obtained in 15 subjects (75%), with an induction time of 26.0 (21.0–33.5)min, and a variation in PEF after sputum induction of −5.9% (−8.9 to 0.0). In one sample, cell count was impossible due to morphological damage. Three patients produced an inadequate sputum sample for processing (2 with sarcoidosis, 1 HP) and 1 patient with sarcoidosis was unable to produce any sputum after 45min induction. The induction was stopped in one subject, due to a 48% PEF fall after 5min of sputum induction.
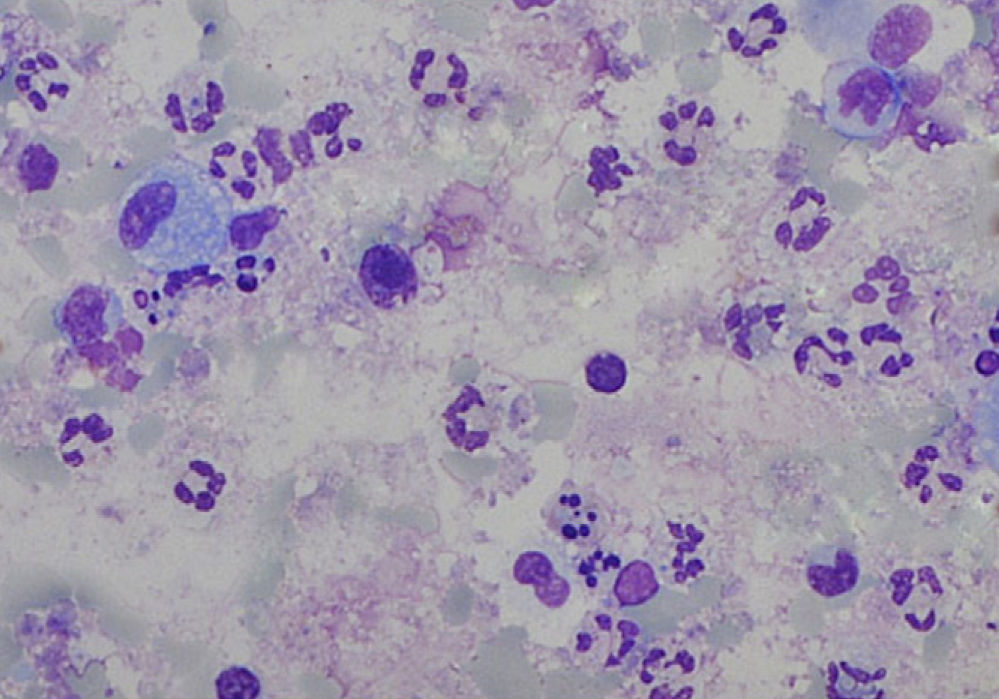
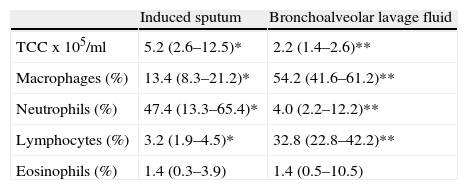
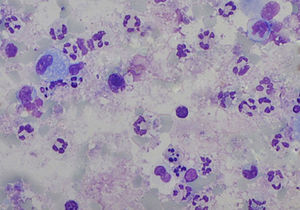
The sputum analysis of the satisfactory samples showed a cellular viability of 55.0% (50.0–63.0), with a total cell count of 5.2 (2.6–12.5)×105/ml, and a predominance of neutrophils (Fig. 1). Total and differential cell counts in IS and BALF are shown in Table 1. Except for eosinophils, all cell counts were significantly different in the two samples (p<0.05). When compared to IS reference values18 our samples showed similar number of total cells counts (median of 1.8×106cells/g vs 2.4×106cells/g) and neutrophil counts (mean 47.4%, expected 36.7%), and an increased percentage of lymphocytes (mean 3.2%, expected 0.5%) and eosinophils (mean 1.4%, expected 0.0%). Similarly, BALF samples in our study group compared to expected values19 had an increase in lymphocytes (33.7 vs <15.0%) and eosinophils (3.8 vs <0.5%) but also a higher percentage of neutrophils (6.9 vs <3.0%).
Total (TCC) and differential cell count in induced sputum (IS) and paired bronchoalveolar lavage fluid (BALF) in the 15 cases with a satisfactory IS sample. Data expressed as median (P25–75) values. Except for eosinophils, all cell counts were significantly different in the two samples.
| Induced sputum | Bronchoalveolar lavage fluid | |
| TCC x 105/ml | 5.2 (2.6–12.5)* | 2.2 (1.4–2.6)** |
| Macrophages (%) | 13.4 (8.3–21.2)* | 54.2 (41.6–61.2)** |
| Neutrophils (%) | 47.4 (13.3–65.4)* | 4.0 (2.2–12.2)** |
| Lymphocytes (%) | 3.2 (1.9–4.5)* | 32.8 (22.8–42.2)** |
| Eosinophils (%) | 1.4 (0.3–3.9) | 1.4 (0.5–10.5) |
*,**Wilcoxon test, p<0.05.
No significant correlations were found between induced sputum and BALF total and differential cell counts in our study group, except for the percentage of neutrophils in Hipersensitivity Pneumonitis (rs=0.9; p=0.05).
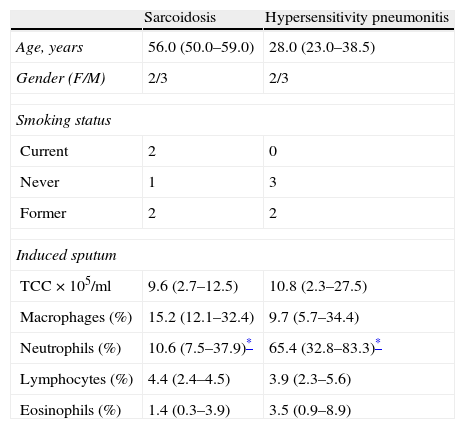
Table 2 presents the sputum analysis in the two patient's subgroups with a lymphocytic alveolitis: sarcoidosis and hypersensitivity pneumonitis. Both pathologies showed similar total cell counts (9.6 vs 10.8×105/ml), and increased lymphocyte numbers (4.4 vs 3.9%). However, significantly higher neutrophil counts were found in HP samples (65.4% vs 10.6%, p<0.05). A similar pattern was seen in BALF samples (9.2% vs 2.8% p<0.05).
Comparison of total (TCC) and differential cell counts in induced sputum in the subgroup of patients with sarcoidosis (n=5) and hypersensitivity pneumonitis (n=5). Data expressed as median (P25–75) values.
| Sarcoidosis | Hypersensitivity pneumonitis | |
| Age, years | 56.0 (50.0–59.0) | 28.0 (23.0–38.5) |
| Gender (F/M) | 2/3 | 2/3 |
| Smoking status | ||
| Current | 2 | 0 |
| Never | 1 | 3 |
| Former | 2 | 2 |
| Induced sputum | ||
| TCC×105/ml | 9.6 (2.7–12.5) | 10.8 (2.3–27.5) |
| Macrophages (%) | 15.2 (12.1–32.4) | 9.7 (5.7–34.4) |
| Neutrophils (%) | 10.6 (7.5–37.9)* | 65.4 (32.8–83.3)* |
| Lymphocytes (%) | 4.4 (2.4–4.5) | 3.9 (2.3–5.6) |
| Eosinophils (%) | 1.4 (0.3–3.9) | 3.5 (0.9–8.9) |
In this study we aimed to assess the safety and feasibility of induced sputum in interstitial lung diseases and to compare the IS cellular findings with those of BALF in newly diagnosed patients. We obtained a satisfactory sputum result with differential cell counts in 15 of the 20 subjects, and the procedure was well tolerated (only one patient experienced a significant PEF fall during the induction).
Although total and differential cell counts in the paired IS and BALF samples were different, confirming that these methods predominantly sample diverse anatomical compartments (respectively, the airways and the deep lung), the results of this pilot study showed that, compared to expected reference values, there was an increased number of lymphocytes and eosinophils in both samples.18,19 In the subgroup of patients with hypersensitivity pneumonitis the relative numbers of neutrophils in induced sputum significantly correlated with BALF counts. Moreover, in these patients neutrophils were significantly increased in both IS and BALF when compared to sarcoidosis (Table 2), another ILD typically occurring with a lymphocytic alveolitis.
Although bronchial hyperreactivity has been recognized in patients with ILD20 and hypertonic saline inhalation can induce bronchoconstriction and cough,21 sputum induction showed itself to be safe in our series, as it has in previous reports.3,5 Only one patient experienced a significant bronchoconstriction during the induction (a 63 years old man, with a suspected HP, pulmonary emphysema and a slight increase in BAL eosinophils of 1.2%).
We obtained a satisfactory sputum sample in 15 subjects (75%). Sobiecka and co-workers3 reported an overall success rate of 56% (33/59 patients with ILD) although with differing success rates between diseases (7/7 in patients with idiopathic pulmonary fibrosis, 11/16 in hypersensitivity pneumonitis and 15/36 in sarcoidosis). These results contrast with data from D’Ippolito et al. that reported a 100% success rate both in sarcoidosis12 and hypersensitivity pneumonitis patients.13 However, to the best of our knowledge, no specific study evaluating the feasibility, safety and functional effects of IS in ILD has been published.6 The sputum analysis of the satisfactory samples we obtained showed a slightly lower cellular viability than that expected for healthy subjects (55% vs 72%), but still within an acceptable range.22
Apart from eosinophils counts, total and differential cell counts in the paired BALF and IS samples were different, suggesting that diverse lung compartments are sampled. A study by Fireman23 reporting segmental lavages at different depths within the airways, showed that the proportion of neutrophils decreased from central (20–30%) to peripheral (<2%) airways, with a corresponding increase in the proportion of macrophages, which suggests that IS derives from larger airways (rich in neutrophils) and BALF samples from more distal airways and alveolar spaces. In support of this hypothesis, we found a significantly higher percentage of neutrophils and a lower percentage of macrophages in IS compared to BALF, as reported in other published series.3,5,12,14,15
As in D’Ippolito et al. studies12,13 no significant correlation was found between the percentage of lymphocytes recovered by both techniques in this study. However, compared to IS reference values,18 our series showed similar total cell counts but with a higher lymphocyte relative count. Immunophenotyping of lymphocyte subsets in IS has been suggested as a complementary method for the diagnosis and monitoring of patients with lymphocytic lung inflammation, such as sarcoidosis. In fact, Fireman et al. reported that a CD4/CD8>2.5 in IS had a positive predicted value of 82% in distinguishing sarcoidosis from other non-granulomatous ILD. Moodley et al.14 and Sobiecka et al.3 also found in sarcoidosis a significant correlation between IS and BALF CD4+/CD8+, in spite of no correlations in the differential cell counts of the two samples. In our series, we also performed flow-cytometry analysis of IS lymphocytes in a limited number of sarcoidosis cases, and we found a CD4+/CD8+>2.5 in 3 of the 4 tested cases (data not shown).
An interesting observation in our small series was that, compared to sarcoidosis, hypersensitivity pneumonitis patients had significantly higher neutrophil count, both in IS and BALF, which correlated significantly in both samples. This possibly reflects the pathological features of hypersensitivity pneumonitis, where increased numbers of neutrophils have been found, especially in recurrent symptomatic forms.24 However, the five patients included had heterogeneous presentations and outcomes, making it difficult to establish any association between the high neutrophil counts and the disease phenotype. One patient had an acute presentation, two subacute and two other chronic presentations. There was only one patient with a fibrous pattern on High Resolution Computed Axial Tomography scan while the other four had mosaic and/or ground glass pattern. In terms of clinical evolution, three patients had disease regression after exposure cessation and corticotherapy, one in a stable chronic form and another had progressive deterioration, dying within two years of the diagnosis.
Our pilot study has some limitations for the generalization of results. Firstly, the studied sample is small and heterogeneous, making it difficult to assess the role of IS in different ILD and so further studies, comparing the inflammatory profiles found in IS and BALF, are needed. However, this population did enable us to evaluate the feasibility and safety of IS in ILD. Secondly, although we have shown that IS in ILD is feasible and well tolerated, the procedure is time-consuming to perform and process and it needs significant technical support, limiting its use to specialized centers.10
The integration of bronchoalveolar lavage results with clinical, radiological, functional and pathological data remains the gold-standard approach for the evaluation of interstitial lung diseases. Although BALF is a minimally invasive technique, with minimal risk under the current guidelines, it cannot be performed in all patients and is costly and questionable in terms of follow-up. We have shown that induced sputum is feasible and safe in this group of patients. Being a less expensive, less invasive and repeatable method, induced sputum is an interesting research and clinical tool in the management of interstitial lung diseases.22,25 Further studies are needed to establish the promising role of induced sputum in the diagnosis and follow-up of interstitial lung diseases.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Araújo, L, et al. Esputo induzido nas doenças pulmonares intersticiais - um estudo piloto. Rev Port Pneumol. 2013. http://dx.doi.org/10.1016/j.rppneu.2012.10.003.