To translate and culturally adapt the Living with Asthma Questionnaire (LWAQ) to the Portuguese language and to test its reliability and validity.
MethodsThe Portuguese version of this disease-specific health-related quality of life measure was obtained with forward/backward translations, consensus panels and a pre-test. The Portuguese LWAQ and Medical Outcomes Study – 36 item Short Form (SF-36) questionnaires, and a form for the characteristics of the patients were administered to 61 subjects with asthma.
ResultsReliability of LWAQ scores was good with Cronbach's alpha coefficients ranging from 0.70 to 0.97 [with the exception of “preoccupation” (0.62) construct, and “sleep” (0.67) and “effects on others” (0.47) domains] and intraclass correlation coefficients between 0.86 and 0.99. Construct validity was supported by the confirmation of predefined hypotheses involving expected significant correlations between LWAQ total, constructs and domains, and SF-36 dimensions with similar content.
ConclusionThe Portuguese LWAQ exhibited suitable psychometric properties, in terms of internal consistency, reproducibility and construct validity.
Traduzir e adaptar culturalmente o Living with Asthma Questionnaire (LWAQ) para a língua portuguesa e testar a sua fiabilidade e validade.
MétodosA versão portuguesa desta medida de qualidade de vida relacionada com a saúde, específica de doença, foi obtida através de traduções e retroversões, painéis de consenso e pré-teste. A versão portuguesa dos questionários LWAQ e Medical Outcomes Study - 36 item Short Form (SF-36), e um formulário das características dos doentes foram administrados a 61 asmáticos.
ResultadosA fiabilidade das pontuações do LWAQ foi considerada boa com coeficientes alfa de Cronbach a variarem entre 0,70 e 0,97 [com exceção do constructo «preocupações» (0,62), e dos domínios «sono» (0,67) e «efeitos nos outros» (0,47)] e coeficientes de correlação intraclasse entre 0,86 e 0,99. A validade de construção foi suportada pela confirmação de hipóteses predefinidas envolvendo as correlações esperadas entre os constructos, domínio e pontuação total do LWAQ, e as dimensões do SF-36 com conceitos semelhantes.
ConclusãoA versão portuguesa do LWAQ apresentou características psicométricas adequadas em termos de coerência interna, reprodutibilidade e validade de construção.
Asthma is one of the most common chronic diseases, affecting an estimated 300 million people worldwide.1 In a study by Sousa et al.,2 the prevalence of asthma in a Portuguese urban population was 10.2%, the mean age of asthmatics was 27.0 years and no significant difference in prevalence was found between males and females. This inflammatory disorder of the airways has an adverse impact on various dimensions of health and quality of life.3 Moreover, the socio-economic impact of asthma is substantial.4 Patient-based instruments are increasingly used to measure the outcomes of health care interventions since they provide evidence of the impact of a specific health condition from the viewpoint of the patient.5
The Living with Asthma Questionnaire (LWAQ)6–8 is a reliable and valid disease-specific health-related quality of life measure which was designed to assess patients with asthma. This questionnaire was developed in the United Kingdom through focus groups discussions and standard psychometric techniques.6–8 In a structured review of the literature on asthma-specific quality of life measures by Apfelbacher et al.,9 the LWAQ was considered the most comprehensive questionnaire.
Validated versions of the LWAQ for the Japanese and10 Norwegian11 languages are already available. However, there was no Portuguese version available before the current study and, in order to apply this questionnaire in Portugal, a process of cross-cultural adaptation and validation was needed. The aim of the present paper is to present the process followed by the authors to translate and culturally adapt the LWAQ to the Portuguese language and to test its reliability and validity in patients with asthma.
MethodsCross-cultural adaptationThe cross-cultural adaptation process of the LWAQ was conducted according to the sequential methodology.12,13 The English LWAQ was translated into Portuguese independently by two Portuguese native translators. The obtained translations were discussed in a first consensus panel to achieve the first preliminary version. This consensus version was translated back to English independently by two English native translators without prior knowledge of the original version. The translations and back translations were discussed in a second consensus panel and reviewed by four physicians (specialists in pneumology and allergology) to achieve a second preliminary version. This consensus version was completed by a panel of 7 patients with asthma (4 females, 3 males; age: 44.0±19.9 years; 3 only can read and write/complete basic education level, 4 complete secondary/superior education level) to verify if all items of the questionnaire were acceptable, understandable, and included all the expected concepts without any redundancy. Patients were selected according to the criteria used in the validation study (as specified in the next section). This panel of patients was held to achieve the final version of Portuguese LWAQ questionnaire.
Validation studySubjectsThe sample comprised consecutive patients with asthma referred for outpatient physical therapy from health care institutions across Castelo Branco during a 5-month period. No attempt was made to standardize the physical therapy treatments. Subjects were selected after obtaining informed consent and checking the inclusion and exclusion criteria. To be included, subjects had to have a diagnosis of asthma validated by a physician, to be aged 18 years or older, and to be referred for physical therapy intervention due to asthma. Subjects were excluded if they had other respiratory disorders or any other disabling problem, or if they were illiterate, not knowing how to read and/or write. All outpatient health care institutions obtained approval from their respective review boards.
MeasurementsMeasurements were performed at the above mentioned outpatient health care institutions. For reproducibility purposes, the subjects who agreed were assessed twice, separated by a 72h interval. This time interval was chosen to minimize the probability of occurrence of relevant changes in patient's clinical condition. Data was collected using the under mentioned patient self-reported measures.
The LWAQ6–8 includes 68 item that produce a total score but can also be combined to cover four constructs (avoidance; distress; preoccupation; activities) and eleven domains (social/leisure; sport; holidays; sleep; work and other activities; colds; mobility; effects on others; medication; sex; dysphoric states). A score, from 0 (very good health-related quality of life) to 2 (very poor health-related quality of life), is separately produced for LWAQ total, constructs and domains.
The SF-3614–16 includes 36 items that are combined in eight subscales: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional and mental health. A score, from 0 (worst possible health status) to 100 (best possible health status), is independently produced for each subscale. The SF-36 was cross-culturally adapted and validated to the Portuguese language.17,18
A form was used to acquire subject information on gender, age and educational level.
Statistical analysesQuantitative variables are described using mean and standard deviation values whereas categorical variables are described using frequency and percentage values.
Reliability. Internal consistency was measured using Cronbach's alpha coefficients. A Cronbach's alpha coefficient between 0.70 and 0.95 received a positive rating.19 Reproducibility of the LWAQ total, constructs and domains was assessed using intraclass correlation coefficients (ICC) for agreement, formula 2.1. An ICC greater than or equal to 0.70 received a positive rating.19
Validity. Construct validity was investigated testing three predefined hypotheses involving expected significant correlations between LWAQ total, constructs and domains, and SF-36 dimensions with similar content: (1) LWAQ total, constructs and domains should present higher number of good to fair (negative) correlations with SF-36 physical functioning, role-physical, general health and vitality than for the other SF-36 dimensions; (2) globally, LWAQ total, constructs and domains should present higher (negative) correlations with SF-36 general health than for the other SF-36 dimensions; (3) LWAQ total, constructs and domains should present higher number of little (negative) or none correlations with SF-36 bodily pain than for the other SF-36 dimensions. Construct validity was analyzed using Spearman's correlation coefficients. Spearman's correlation coefficients were interpreted as follows: excellent relationship if higher than 0.90; good if between 0.90 and 0.71; fair if between 0.70 and 0.51; weak if between 0.50 and 0.31, little or none if lower than or equal to 0.30.20 A p value of 0.05 was taken as the reference level of significance.
Statistical analyses were performed using SPSS 15.0 for Windows.
ResultsCross-cultural adaptationThe second preliminary version of the Portuguese LWAQ questionnaire was well accepted in the pre-test. All the questions and response options were considered satisfactorily understandable by the subjects. Even so, in order to improve clarity, minor rewording was made on three items, based on patient's suggestions. On the item 9 the sentence “o meu corpo irrita-me” (Pt), previously chosen as translation of “I feel angry with my body”, was reworded to “o meu corpo incomoda-me” (Pt). On the items 28 and 37 the term “um monte” (Pt), previously chosen as translation of “a hill”, was reworded to “uma ladeira/subida” (Pt). The revised version was used in the validation study.
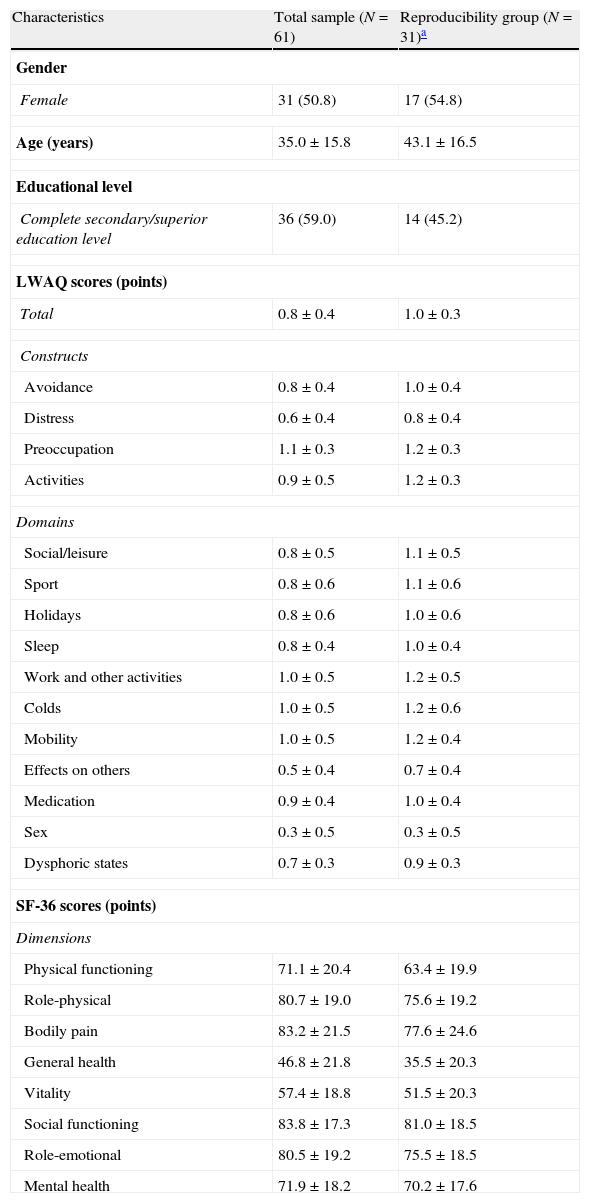
Validation studySubjectsThe descriptive statistics are presented in Table 1. A total of 61 patients were included in the internal consistency and construct validity assessment, of which 31 (50.8%) were also included in the reproducibility assessment. All patients had the disease controlled.
Characteristics of the subjects.
| Characteristics | Total sample (N=61) | Reproducibility group (N=31)a |
| Gender | ||
| Female | 31 (50.8) | 17 (54.8) |
| Age (years) | 35.0±15.8 | 43.1±16.5 |
| Educational level | ||
| Complete secondary/superior education level | 36 (59.0) | 14 (45.2) |
| LWAQ scores (points) | ||
| Total | 0.8±0.4 | 1.0±0.3 |
| Constructs | ||
| Avoidance | 0.8±0.4 | 1.0±0.4 |
| Distress | 0.6±0.4 | 0.8±0.4 |
| Preoccupation | 1.1±0.3 | 1.2±0.3 |
| Activities | 0.9±0.5 | 1.2±0.3 |
| Domains | ||
| Social/leisure | 0.8±0.5 | 1.1±0.5 |
| Sport | 0.8±0.6 | 1.1±0.6 |
| Holidays | 0.8±0.6 | 1.0±0.6 |
| Sleep | 0.8±0.4 | 1.0±0.4 |
| Work and other activities | 1.0±0.5 | 1.2±0.5 |
| Colds | 1.0±0.5 | 1.2±0.6 |
| Mobility | 1.0±0.5 | 1.2±0.4 |
| Effects on others | 0.5±0.4 | 0.7±0.4 |
| Medication | 0.9±0.4 | 1.0±0.4 |
| Sex | 0.3±0.5 | 0.3±0.5 |
| Dysphoric states | 0.7±0.3 | 0.9±0.3 |
| SF-36 scores (points) | ||
| Dimensions | ||
| Physical functioning | 71.1±20.4 | 63.4±19.9 |
| Role-physical | 80.7±19.0 | 75.6±19.2 |
| Bodily pain | 83.2±21.5 | 77.6±24.6 |
| General health | 46.8±21.8 | 35.5±20.3 |
| Vitality | 57.4±18.8 | 51.5±20.3 |
| Social functioning | 83.8±17.3 | 81.0±18.5 |
| Role-emotional | 80.5±19.2 | 75.5±18.5 |
| Mental health | 71.9±18.2 | 70.2±17.6 |
Quantitative variables: mean±standard deviation; Categorical variables: frequency (percentage).
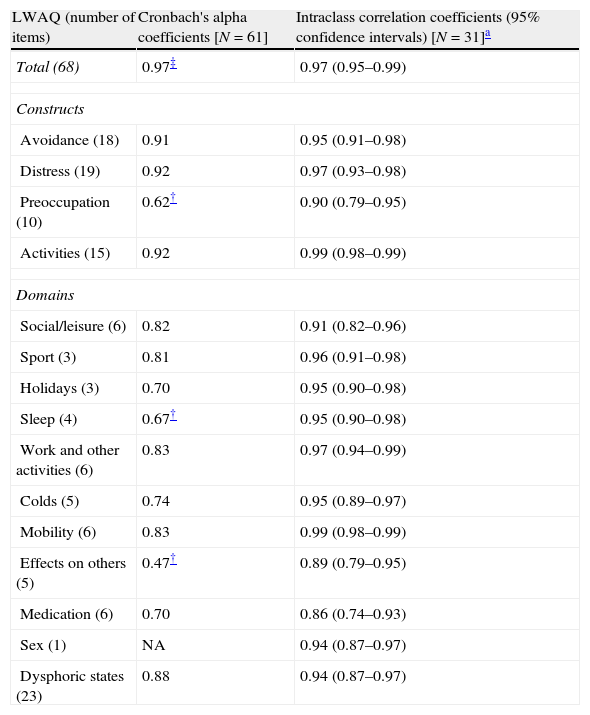
All of the reliability results are summarized in Table 2.
Reliability of the LWAQ total, constructs and domains.
| LWAQ (number of items) | Cronbach's alpha coefficients [N=61] | Intraclass correlation coefficients (95% confidence intervals) [N=31]a |
| Total (68) | 0.97‡ | 0.97 (0.95–0.99) |
| Constructs | ||
| Avoidance (18) | 0.91 | 0.95 (0.91–0.98) |
| Distress (19) | 0.92 | 0.97 (0.93–0.98) |
| Preoccupation (10) | 0.62† | 0.90 (0.79–0.95) |
| Activities (15) | 0.92 | 0.99 (0.98–0.99) |
| Domains | ||
| Social/leisure (6) | 0.82 | 0.91 (0.82–0.96) |
| Sport (3) | 0.81 | 0.96 (0.91–0.98) |
| Holidays (3) | 0.70 | 0.95 (0.90–0.98) |
| Sleep (4) | 0.67† | 0.95 (0.90–0.98) |
| Work and other activities (6) | 0.83 | 0.97 (0.94–0.99) |
| Colds (5) | 0.74 | 0.95 (0.89–0.97) |
| Mobility (6) | 0.83 | 0.99 (0.98–0.99) |
| Effects on others (5) | 0.47† | 0.89 (0.79–0.95) |
| Medication (6) | 0.70 | 0.86 (0.74–0.93) |
| Sex (1) | NA | 0.94 (0.87–0.97) |
| Dysphoric states (23) | 0.88 | 0.94 (0.87–0.97) |
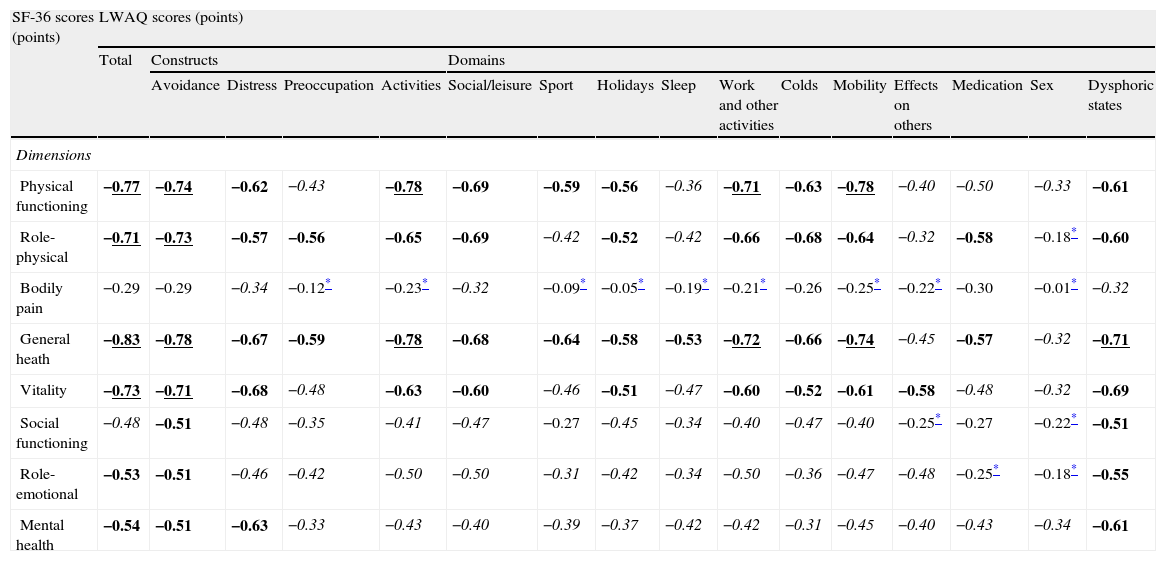
The three predefined hypotheses concerning construct validity were confirmed (Table 3).
Construct validity of the LWAQ total, constructs and domains (N=61).
| SF-36 scores (points) | LWAQ scores (points) | |||||||||||||||
| Total | Constructs | Domains | ||||||||||||||
| Avoidance | Distress | Preoccupation | Activities | Social/leisure | Sport | Holidays | Sleep | Work and other activities | Colds | Mobility | Effects on others | Medication | Sex | Dysphoric states | ||
| Dimensions | ||||||||||||||||
| Physical functioning | −0.77 | −0.74 | −0.62 | −0.43 | −0.78 | −0.69 | −0.59 | −0.56 | −0.36 | −0.71 | −0.63 | −0.78 | −0.40 | −0.50 | −0.33 | −0.61 |
| Role-physical | −0.71 | −0.73 | −0.57 | −0.56 | −0.65 | −0.69 | −0.42 | −0.52 | −0.42 | −0.66 | −0.68 | −0.64 | −0.32 | −0.58 | −0.18* | −0.60 |
| Bodily pain | −0.29 | −0.29 | −0.34 | −0.12* | −0.23* | −0.32 | −0.09* | −0.05* | −0.19* | −0.21* | −0.26 | −0.25* | −0.22* | −0.30 | −0.01* | −0.32 |
| General heath | −0.83 | −0.78 | −0.67 | −0.59 | −0.78 | −0.68 | −0.64 | −0.58 | −0.53 | −0.72 | −0.66 | −0.74 | −0.45 | −0.57 | −0.32 | −0.71 |
| Vitality | −0.73 | −0.71 | −0.68 | −0.48 | −0.63 | −0.60 | −0.46 | −0.51 | −0.47 | −0.60 | −0.52 | −0.61 | −0.58 | −0.48 | −0.32 | −0.69 |
| Social functioning | −0.48 | −0.51 | −0.48 | −0.35 | −0.41 | −0.47 | −0.27 | −0.45 | −0.34 | −0.40 | −0.47 | −0.40 | −0.25* | −0.27 | −0.22* | −0.51 |
| Role-emotional | −0.53 | −0.51 | −0.46 | −0.42 | −0.50 | −0.50 | −0.31 | −0.42 | −0.34 | −0.50 | −0.36 | −0.47 | −0.48 | −0.25* | −0.18* | −0.55 |
| Mental health | −0.54 | −0.51 | −0.63 | −0.33 | −0.43 | −0.40 | −0.39 | −0.37 | −0.42 | −0.42 | −0.31 | −0.45 | −0.40 | −0.43 | −0.34 | −0.61 |
Spearman's correlation coefficients (LWAQ is 0–2 points, best to worst; SF-36 is 0–100 points, worst to best).
Good correlations in bold/underline; fair correlations in bold; weak correlations in italic; little or none correlations in regular.
The cross-cultural adaptation process was conducted successfully and resulted in a reasonably acceptable and understandable Portuguese version of the LWAQ.
High Cronbach's alpha coefficients for most of the scores of the measure confirmed that the Portuguese LWAQ total, constructs (except preoccupation) and domains (except sleep and effects on others) are internally consistent. Even the LWAQ total, which was above the acceptable value of 0.95, yielded a Cronbach's alpha coefficient of 0.97. Similar internal consistency findings were obtained by the Norwegian version of the LWAQ (Cronbach's alpha coefficient of 0.97).11
High ICC for all the scores of the measure revealed that the stability of the Portuguese LWAQ total, constructs and domains over time was good. Similar reproducibility findings were obtained by the original version of the LWAQ (r=0.956 and r=0.97), by the Japanese version (r=0.81)10 and by the Norwegian version (r=0.95).11
All predefined hypotheses involving expected significant correlations between LWAQ total, constructs and domains, and SF-36 dimensions (with similar content) were confirmed. In fact, LWAQ scores presented better correlations with the SF-36 dimensions that contribute to the physical component, in particular the general health dimension, than for the mental component. The exception was the bodily pain dimension, which is justified by the fact that pain is not the main symptom in asthma. Other studies also provided evidence for construct validity of the LWAQ as indicated by factor analysis8,10, known-groups comparisons6,11, and associations with other existing measures7,11.
Some limitations of this study should be acknowledged. The sample used is not representative of the entire population of Portuguese patients with asthma. In fact, only patients with asthma referred for physical therapy were recruited. Further validation in other asthma populations is therefore advised. Owing to practical reasons, asthma severity and duration were not record. Further validation studies should consider these variables. The responsiveness of the Portuguese LWAQ was not tested. More testing is needed in order to test this psychometric property.
Nevertheless, we may conclude that the Portuguese LWAQ evidenced good reliability and validity for patients with asthma.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank the physical therapy staff from the outpatient health care institutions. In addition, the patients who participated in this study also deserve our deep gratitude.
Please cite this article as: Soles Gonçalves R, et al. Adaptação cultural e validação da versão portuguesa do Living with Asthma Questionnaire. Rev Port Pneumol. 2013. http://dx.doi.org/10.1016/j.rppneu.2013.02.002