Chronic obstructive pulmonary disease (COPD) is one of the main causes of death and disability worldwide. Many treatment options are now available, but criteria for choosing inhaled bronchodilators and inhaled corticosteroids have been under discussion. New trials have highlighted the role of patient`s characteristics, such as eosinophil count and exacerbation history, in selecting the most effective personalised treatment option.
MethodsIn this conceptual review, an in-depth rationale is developed with an integrative approach to COPD treatment, gathering data from the main clinical trials performed so far and that may provide support for actual GOLD 2023 recommendations.
ResultsAccording to the patient's characteristics and profile, different treatment options, including mono, dual and triple therapies, are presented in a diagram matrix, comparing their efficacy in terms of reduction of exacerbations and mortality risk.
Discussion and conclusionEosinophil counts and past exacerbation profile may play equally relevant roles to predict the individual risk and the potential response to inhaled corticosteroids. Thus, a comprehensive approach considering these two predictors is needed to aid clinicians decide preventative actions and choice of a first-line or step-up treatment.
Chronic obstructive pulmonary disease (COPD) is one of the main causes of death and disability worldwide, affecting 380 million people according to the World Health Organization (WHO)1, and its prevalence is predicted to rise, considering the ageing population. Many patients with COPD show poor clinical control, being at risk of exacerbations and death.2 The main objective of treating these patients is to prevent lung function decline and future exacerbations. Inhaler therapy with bronchodilator drugs is the most widely used treatment, allowing symptom control and reduction of risk of exacerbations and death, but some patients also need combinations with inhaled corticosteroids.3 However, most patients do not adhere to their regimes, and do not use their inhalers correctly, which contributes to poor outcomes.4–7
Many treatment options are now available, involving either inhaled bronchodilator drugs or inhaled corticosteroids, but phenotypic heterogeneity of patients highlights the need for personalised care.3 In recent years, many important changes have been reflected in clinical guidelines, mainly regarding moderate to severe stages of COPD; this is due to the contribution of major trials such as the IMPACT trial, and more recently, the ETHOS trial.8,9 These trials yielded new evidence regarding the role of dual therapies (either in a long-acting beta-agonist (LABA) plus long-acting muscarinic antagonist (LAMA) association or as LABA plus inhaled corticosteroids (ICS) combination), as well as the role of LABA/LAMA/ICS triple-therapy in such patients. The IMPACT trial was the first to show a significant reduction of mortality risk as a hard endpoint, which is a key ground-breaking step in COPD research. In addition, with its subsequent results10,11, criteria for choosing inhaled bronchodilators and inhaled corticosteroids (ICS) have become clearer, basing the decision on the patient`s profile and characteristics, such as eosinophil counts and exacerbation history.3 Based on these recent trials, the importance of using hard outcomes, such as exacerbations and mortality risk, is growing and deserves further research.
Recently, the Global Initiative for COPD (GOLD) released their 2023 document, which includes two main changes, namely: 1) the initial treatment for GOLD B patients, which is now recommended with LABA/LAMA dual-therapy; 2) a new GOLD E group, resulting from merged GOLD C and D groups, with initial treatment recommended with LABA/LAMA dual-therapy, or LABA/LAMA/ICS triple-therapy for those with eosinophil counts over 300 cells/mm3.3 Nevertheless, an in-depth rationale sustaining these new changes is lacking in the GOLD report.
Thus, we developed a comprehensive review, gathering data from the major trials performed so far, namely the ones reporting exacerbation and mortality risk as endpoints, in order to provide an integrative rationale for the COPD treatment recommendations, according to the patient's characteristics and profile.
MethodsWe carried out a critical and conceptual review, following the recommendations from Grant et al. published in Health Information and Library Journal.12
Searches were conducted in MEDLINE and CENTRAL for papers reporting clinical trials in COPD. A combination of “Pulmonary Disease, Chronic Obstructive” MeSH term with “mortality” or “exacerbation” was used in the queries, covering a period from inception until August 2021. The criteria for study selection were: clinical trial, addressing inhaled drug treatment in COPD patients, either comparing different treatment options or active treatments versus placebo, and reporting hard clinical outcomes such as exacerbation and mortality risk. Studies reporting only lung function, symptoms, or quality of life as the main outcomes were not considered, as they fall out the scope of this review, which aims to address the efficacy of COPD therapies regarding hard outcomes.
Papers were selected according to the established criteria and appraised by two independent reviewers. Data from participants’ characteristics, study design features, as well as from outcome effects were collected to perform a critical appraisal between studies. However, considering the conceptual nature of this review, no objective and systematic method was used to analyse the quality of the included studies, nor was any analytical method applied to compare their results.
ResultsSixteen relevant trials published in the last 15 years fell within the scope of this review: IMPACT8, ETHOS9, EMAX13, TRIBUTE14, SUNSET15, TRINITY16, FLAME17, TRILOGY18, SPARK19, INSPIRE20, TORCH21, SUMMIT22, DYNAGITO23, POET-COPD24, UPLIFT25 and the one published by Welte et al.26 All of these trials were randomised, single or double blinded, comparing different therapies for COPD (mono versus dual vs triple) and reporting exacerbation rates or mortality as outcomes of interest.
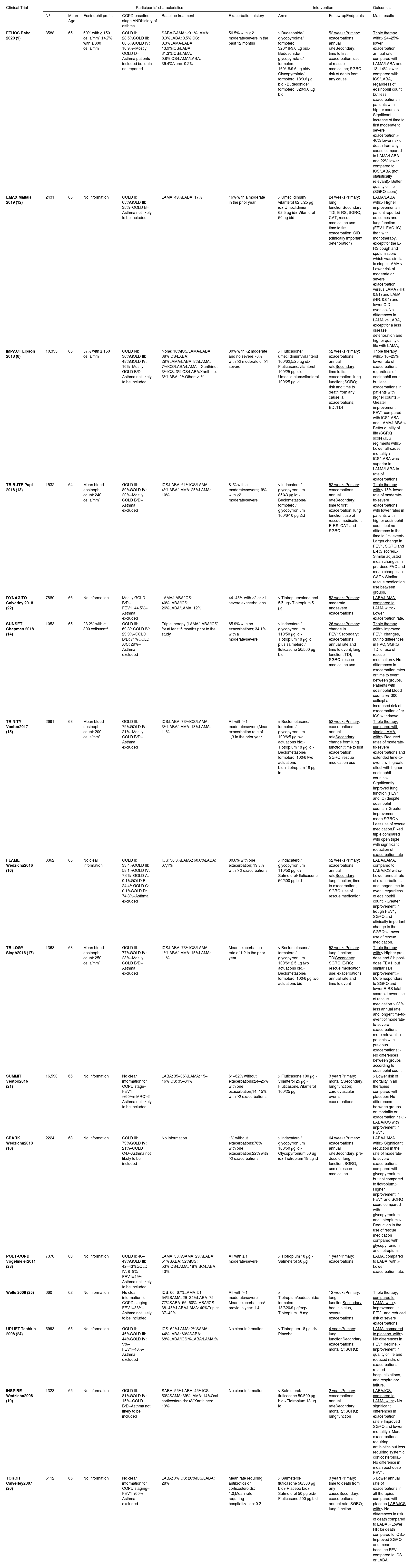
Table 1 presents the main characteristics of the most relevant trials reporting COPD drug treatment data. No study has considered environmental and occupational exposures, although they are relevant factors that impact hard outcomes of interest.27,28
Clinical trials reporting the efficacy of COPD treatment on exacerbations and mortality risk and their main characteristics.
CAT - COPD Assessment Test; COPD - Chronic Obstructive Lung Disease; E-RS - EXACT-Respiratory Symptoms; FEV1 - forced expiratory volume in the first second; FVC - forced vital capacity; GOLD - Global Initiative for Chronic Obstructive Lung Disease; HR - hazard ratio; IC - inspiratory capacity; ICS - Inhaled Corticosteroids; LABA - Long Acting Beta Agonist; LAMA - Long Acting Muscarinic Antagonist; mMRC - modified Medical Research Council dyspnoea scale; SABA - Short Acting Beta Agonist; SAMA - Short Acting Muscarinic Antagonist; SGRQ - Saint George's Respiratory Questionnaire; TDI - Transition Dyspnoea Index.
Analyses of the features and characteristics of these trials identified some aspects which should be highlighted:
- •
Most studies have included participants of similar age (mean values of 65 years) with adequate follow-up times to provide appropriate outcome estimates (most up to 2–3 years).
- •
In the majority of studies, asthma patients were excluded or, at least, not likely to be included, except for ETHOS9 where some asthma patients were included, but exact data were not reported.
- •
Exacerbation rates, clinical control, quality of life and lung function were the most frequently reported outcomes, although some studies sought to estimate the impact on respiratory and all-cause mortality, but only as secondary outcomes8,9,20,21, with the exception of TORCH.21 ETHOS9 and IMPACT.8 studies were the most recent ones to address mortality risk and found significant benefit of triple therapies, with reductions of up to 40% in mortality relative risk. The EMAX13 study addressed a new concept, not frequently used in such trials, which is a global assessment of disease severity, defined by the “clinically important deterioration” (CID) composite endpoint. CID is defined by a deterioration from baseline in individual patients in terms of forced expiratory volume in one second (FEV1), and/or in any patient-reported outcomes and/or the occurrence of a moderate or severe COPD exacerbation.
- •
Most studies included COPD patients in GOLD B and D stages (predominantly highly symptomatic patients), except for SPARK19, which included a relevant proportion of patients in GOLD C stage (less symptomatic but with previous exacerbation history). No clear information was found to categorise patients in the TORCH21 and SUMMIT22 studies.
- •
Some studies included participants with a history of higher number/intensity of previous exacerbations (such as ETHOS9, IMPACT8, TRIBUTE14, DYNAGITO23, TRINITY16 and SPARK19): for those, the benefit of ICS combination therapies was higher; on the other hand, studies with participants with fewer previous exacerbations (such as SUNSET15, EMAX13, FLAME17 and SUMMIT22) showed greater benefit with LABA/LAMA therapies.
- •
SUNSET15, IMPACT8 and ETHOS9 trials allowed a clear discrimination of participant's blood eosinophil levels. We can assume that the presented mean blood eosinophil counts in the TRIBUTE (240 cells/mm3)[14], TRINITY (200 cells/mm3)[16] and TRILOGY (250 cells/mm3)[18] trials also represent a majority of patients with elevated blood eosinophil counts (>150 cells/ mm3), compared with the remaining ones. However, SUNSET15 had a shorter follow-up period and included participants with low exacerbation profile, while IMPACT8 and ETHOS9 included participants with higher exacerbation history.
- •
FLAME17 revealed no differences in exacerbation rates, regarding ICS response to different levels of blood eosinophils count, but most of the included participants had a low profile of previous exacerbations.
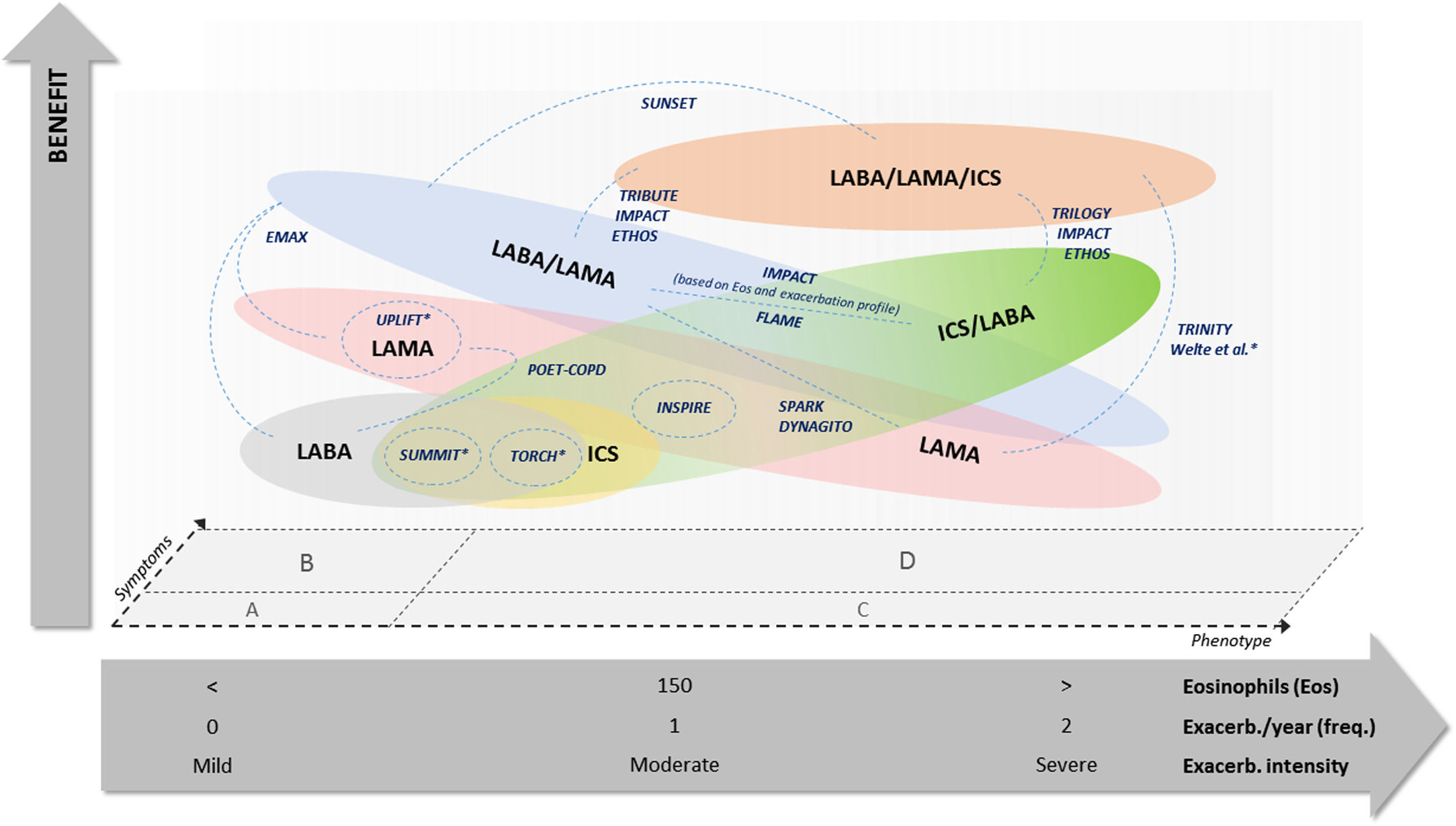
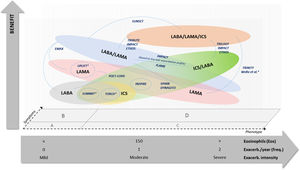
Considering such aspects, we elaborated an original conceptual diagram comparing the potential benefit of different options of inhaled treatment for COPD in reducing the risk of exacerbation and mortality (Fig. 1). The diagram positions the main trials conducted so far according to their reported results and the most relevant patients’ characteristics. Apart from such features, critical judgement may also include a third dimension, i.e. symptom intensity, which might influence the choice of different drug combinations with similar potential benefits.
Comparison of potential benefit of COPD treatment options on exacerbation and mortality risk reduction, according to clinical trials results and patients’ characteristics. Notes: X-axis shows a concept of integrative clinical judgement on patients’ phenotypic features, but positioning of such different aspects is not truncated and may suffer overlapping adjustments. Studies are located according to their participants’ features in order to estimate their potential comparisons. Most studies are situated in GOLD B and D symptoms regions, while SPARK study is situated in GOLD C and D symptoms regions. *Studies with unclear participants’ profile, either regarding eosinophil count or COPD symptoms group.
This figure is original, produced by authors according to their conceptual rationale, and reproduced with permission to journal policies.
Thus, when considering the risk of exacerbation and mortality, there may be greater benefit of dual bronchodilator therapy (LABA plus LAMA) over single therapy (LABA or LAMA) in patients at GOLD stages A and B (mainly in patients with persistent mild or moderate symptoms, but with lower blood eosinophil counts and without previous exacerbations). Alternatively, when considering a single bronchodilator therapy, LAMA should be preferred over LABA, as it may have greater benefits in terms of preservation of lung function.
Regarding patients with higher blood eosinophil counts and a relevant history of past exacerbations, either in frequency or in intensity, triple therapy (LABA plus LAMA plus ICS) should be considered over any dual therapies, as it is more beneficial in preventing exacerbations and mortality; this includes mostly patients at (previous) GOLD stages C and D. As an alternative, whenever triple therapy is not a feasible choice or unavailable, dual therapy with LABA/ICS should be an option to consider, particularly in patients with higher blood eosinophils count that will respond better to the ICS.
DiscussionThe role of COPD patients’ phenotypes has been under discussion over the last few years in light of new evidence that major trials have revealed. GOLD reports have been changing significantly, including new insights into the role of blood eosinophil counts and exacerbation profile in clinical decision making3, mostly in high-risk patients and concerning the choice of drug combinations, including or not the ICS. Indeed, it is important to note that an observational study in different Italian areas, in which 176 general practitioners (GPs) enroled their patients with a COPD diagnosis (n = 526), found a higher prescriptive appropriateness when using the 2011 GOLD classification, with respect to the previous GOLD classifications.29 This might be because the 2011 GOLD classification, unlike the old ones, included common anamnestic features considered by GPs in their clinical practice.
Some studies have highlighted the importance of blood eosinophil counts as an independent predictor for individual risk regarding exacerbations and mortality.11,30 However, looking at patients’ profiles in the studies, we can figure out that blood eosinophil counts and past exacerbation profile may be equally relevant to trace individual risk and potential response to ICS. For instance, SUNSET15, IMPACT8 and ETHOS9 studies had many participants with high blood eosinophil counts. Nevertheless, SUNSET15 participants had a lower exacerbation profile, probably related to triple-therapy effects prior to the study (and thus, ICS therapies), which allowed the discontinuation of ICS during the study, thereby precluding major differences compared with ICS-free therapeutic schemes. On the other hand, IMPACT8 and ETHOS9 included participants with higher exacerbation profiles, and therefore the benefit of triple-therapy was higher. Thus, the frequency and intensity of past exacerbations may, to some extent, overcome the role of eosinophil counts in predicting ICS response. This may explain the results found in the FLAME study17, where patients had a lower exacerbation profile, and no differences in the outcome were found along the spectrum of eosinophil levels. These results may provide a solid rationale to position the potential benefit for triple therapy on those patients.
Triple-therapy has come under the spotlight over the last few years, mostly due to the IMPACT8 results, that, for the first time in COPD drug therapy research, found a statistically significant reduction of mortality risk. The higher the individual risk patients presented the more pronounced were the results, mainly regarding eosinophil levels or the frequency and severity of previous exacerbations. Such findings were reinforced by the ETHOS9 trial and by real-world studies.31 The benefits of triple-therapy may extend to quality of life and lung function itself as reported by most of these trials32–34, although this has not been proven in real life practice in the long term, thus far. However, it should be highlighted that mortality was assessed as a secondary outcome in most trials, and their sample size was not powered to adequately allow this analysis. Thus, future trials should be designed with mortality as the main outcome. Furthermore, this overall mortality reduction seems to occur despite a slight but statistically relevant increase in pneumonia and other ICS related adverse effects. Nevertheless, the potential benefit regarding exacerbation should be balanced with the risk of pneumonia.35
The EMAX study13, on the other hand, brought new evidence regarding the superiority of dual bronchodilator therapy versus single therapy in mild stages of COPD. However, there were insufficient data regarding eosinophil counts, which limits the possibility of assuming its potential benefit when comparing these two treatment options. Moreover, most patients were selected from a GOLD B symptoms spectrum, and no study has yet addressed GOLD A (less symptomatic) patients, which hampers the ability to conclude about the superiority of dual therapy over single therapy in such patients.
Most of the trials included in our analysis involved patients in GOLD B and (previous) D stages, except for SPARK19, which had a significant proportion of GOLD (previous) C patients. The latter present fewer symptoms but have a higher exacerbation profile. SPARK revealed a less robust benefit of dual therapy, but the true magnitude of the ICS benefit in the previous GOLD C group patients is still unclear and deserves further research.
Patients with higher eosinophil counts and/or a history of previous moderate-severe exacerbations seem to benefit more from ICS containing therapies. Nevertheless, the true determining magnitude of each predictor, and whether one plays a more important role than the other, is still unclear. Based on these findings, new approaches are now recommended by GOLD guidelines3, namely:
- •
Dual therapy with LABA/LAMA as the first-line treatment for GOLD B patients, without previous exacerbations and despite the intensity of symptoms. Those patients most likely have low eosinophil levels and therefore get a less clear benefit with LABA/ICS combination.
- •
Update on patients with previous exacerbations, with the development of a new GOLD E group, resulting from the merged (previous) groups C and D. Those patients should be initiated with LABA/LAMA dual-therapy, as was previously stated. However, those with higher eosinophil counts will benefit the most from inclusion of the ICS to prevent future exacerbations, and as an add-on to bronchodilator therapy to preserve lung function. As an alternative, dual therapy with LABA/ICS should be an option to consider in those patients, whenever triple therapy is not a feasible choice or is unavailable.
- •
A step-up treatment, for patients starting on single-bronchodilator therapy, directly to triple-therapy, particularly in the presence of high eosinophil counts and frequent moderate or severe exacerbations. Those patients will benefit the most with the presence of the ICS to prevent future exacerbations. Moreover, considering that they are frequent (or intense) “exacerbators”, they have a higher risk of lung function decline; therefore the choice of giving them a combination of LABA and LAMA is potentially better than each one isolated, to preserve lung function.
It seems reasonable to consider that all patients diagnosed with COPD benefit most from an initial combined therapy, either a LABA/LAMA for patients without previous exacerbations but with persistent symptoms (mostly GOLD B, but possibly some at higher risk in GOLD A), a LABA/LAMA for patients with previous exacerbations, and maybe a LABA/LAMA/ICS for those with higher blood eosinophil counts (GOLD E). Moreover, a step-up therapy approach should lead to triple therapy in the presence of frequent or severe exacerbations or higher blood eosinophil counts. This approach will probably optimise the potential benefit to patients in terms of preventing lung function decline, exacerbations, and mortality risk. However, it may not be reasonable and feasible in all settings, especially in low and middle-income countries, where access to combined therapies may be limited due to lack of supply or economic constraints. Moreover, other aspects such as patient preference for different types of inhalers, treatment adherence, a patient's ability to acquire and perform proper inhalation manoeuvres, or even doctors’ adaptability to different inhaler treatment features, may all play a role in the final treatment choice. For that reason, alternative approaches may prevail in certain circumstances, such as a single bronchodilator initial therapy for GOLD A and B patients (mainly at a lower individual risk, such as non-smokers, healthy and physically active, etc.), or even dual therapy with LABA/ICS for patients in GOLD E group.
This review focuses on the main patient characteristics reported in clinical trials, such as clinical staging, exacerbation history and blood eosinophil counts. However, grey areas remain about which is the best drug combination for each specific patient, considering the multidimensional matrix of such features. This limitation, however, is the reason why the present paper puts forward a conceptual exercise, positioning different drug options and combinations, regarding their potential benefit in the risk of exacerbations and mortality as a misbalancing factor in clinical decision. Another aspect that may hamper the objectiveness of this review concerns the differences amongst specific drugs, even within each class, which we did not directly address, and which has been reported for instance in the bronchodilator effect of LAMA and the pharmacodynamics of ICS.36,37
A relevant topic in bronchodilation in COPD involves the side effects of the different molecules, which we did not specifically take into account in the formulation of the clinical decision conceptual diagram. This question has been studied, especially regarding ICS containing inhalers and the increased risk of pneumonia.38 Some of the most recent reviews consider that the all-cause mortality risk reduction outweighs the risk of pneumonia with ICS.39 Since we developed this rationale over the existing GOLD framework, which takes into consideration the risk-benefit of inhaled bronchodilators, and that recent evidence favours the benefits of most of the referred bronchodilators over the risks, we do not consider this limitation as a drawback to the conclusions that led us to the conceptual diagram.
Nevertheless, we should highlight that this is a critical and conceptual review, as no systematic methods were used to address our research question/hypothesis or to search and select the included studies.12 In addition, the lack of an objective and systematic method to analyse the results and the quality of the included studies may be regarded as a limitation. However, we wish to point out that our critical review may contribute to the discussion initiated in the respiratory community after the release of the new GOLD 2023 document. We envisage new systematic reviews conducted with analytical methods to compare clinical trials results, and to further enrich these results with observational data. That might help to establish quantitatively if eosinophil counts and past exacerbation profile play equally relevant roles to predict ICS response.
Future trials should focus on mortality as the primary endpoint, but also try to clarify the role of patients’ phenotypic features, such as exacerbation history, eosinophil levels, and many others, at all COPD stages and across disease progression spectra.
Particular attention should be paid to the ubiquitous environmental exposures, which, according to the World Health Organization (WHO) are responsible for 13 million anticipated deaths per year worldwide, including more than seven million people who are killed each year from exposure to air pollution.40 Indeed, all clinicians dealing with chronic respiratory patients should know the importance of air pollution also for the hard outcomes of interest27 and manage the issue both at the level of doctor-patient relationship and at the community level as clean air advocates.41–43
Of particular interest, we suggest that a new COPD research agenda regarding inhaled therapy should focus on the long-term outcomes of therapy naïve patients initiating dual or triple therapy inhalers, considering exacerbations, COPD mortality and all-cause mortality, but also lung function preservation and clinically important deterioration. Studies should also focus on the comparison of single to triple therapies step-ups in terms of exacerbations and mortality risk reduction, using the time-to-event endpoints. Finally, a debate is ongoing on the definition and severity classification of exacerbations, as well as their predictive potential for other future exacerbations. Thus, studies are needed to also address those aspects, as they may ultimately change the phenotype classification and treatment of COPD patients.44,45
ConclusionThe role of patients’ phenotypic features has been changing in the last few years. New trials have shown the importance of eosinophil counts and past exacerbation profile to predict the individual risk and the potential benefit of ICS containing therapies. The conceptual review here presented offers a comprehensive approach that considers patients’ main phenotypic features and the potential benefit of different COPD drug treatment options. Moreover, this may, to some extent, provide a possible rationale for the choice of the first-line option and of the step-up treatment with dual therapies containing or not ICS, or even a step-up directly from monotherapy to triple therapy.
Statement of ethicsThis review was developed under the ethical principles. No human participation was necessary, and no personal data was used, therefore no ethical committee appraisal was conducted.
Author contributionsAll authors contributed to all stages of the manuscript development, namely: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
None to declare.