There is currently no consensus on the treatment sequence in chronic obstructive pulmonary disease (COPD), although it is recognized that early diagnosis is of paramount importance to start treatment in the early stages of the disease. Although it is fairly consensual that initial treatment should be with an inhaled short-acting beta agonist, a short-acting muscarinic antagonist, a long-acting beta-agonist or a long-acting muscarinic antagonist. As the disease progresses, several therapeutic options are available, and which to choose at each disease stage remains controversial. When and in which patients to use dual bronchodilation? When to use inhaled corticosteroids? And triple therapy? Are the existing non-inhaled therapies, such as mucolytic agents, antibiotics, phosphodiesterase-4 inhibitors, methylxanthines and immunostimulating agents, useful? If so, which patients would benefit? Should co-morbitities be taken into account when choosing COPD therapy for a patient?
This paper reviews current guidelines and available evidence and proposes a therapeutic scheme for COPD patients. We also propose a treatment algorithm in the hope that it will help physicians to decide the best approach for their patients. The authors conclude that, at present, a full consensus on optimal treatment sequence in COPD cannot be found, mainly due to disease heterogeneity and lack of biomarkers to guide treatment. For the time being, and although some therapeutic approaches are consensual, treatment of COPD should be patient-oriented.
One of the main reasons that has precluded the establishment of a consensus treatment sequence in chronic obstructive pulmonary disease (COPD) is that patient stratification is not consensual across guidelines.1–4 The first problem emerges with the classification by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) only distinguishing two issues, symptoms and risk,1 which are clearly not sufficient to define a patient with COPD, an extremely heterogeneous disease. The Spanish guidelines propose treatment based on four clinically relevant phenotypes,2 but even this approach presents heterogeneity. Although it is fairly consensual that initial treatment should begin with an inhaled short-acting beta agonist (SABA), a short-acting muscarinic antagonist (SAMA), a long-acting beta-agonist (LABA) or a long-acting muscarinic antagonist (LAMA),1,2,4,5 when and in which patients to use dual bronchodilation, inhaled corticosteroids (ICS) in combination with bronchodilation, or triple therapy, remains controversial,6 except in patients with the Asthma-COPD overlap syndrome (ACOS), who should be initially treated with ICS.7 ICS have been shown to have side effects, namely pneumonia,6 and tuberculosis and the risk increases dose-dependently with ICS use.8
Apart from the above mentioned molecules, other potentially useful non-inhaled therapies such as mucolytic agents, antibiotics, phosphodiesterase-4 inhibitors (PDE4i), methylxanthines and immunostimulating agents, are available, but again their use is not consensual.1–4 Although increasingly relevant and important to the management of COPD, this paper does not include a section on non-pharmacological measures, which are duly addressed by Guimarães et al.9
This paper reviews current guidelines and available evidence and proposes a therapeutic scheme for COPD patients.
MethodsThe GI DPOC-Grupo de Interesse na Doença Pulmonar Obstrutiva Crónica convened on December 2014 to review and discuss previously distributed papers, scientific meeting presentations, current guidelines and practices related to the management of COPD. Papers had been identified through searching PubMed with the search terms “chronic obstructive pulmonary disease dual bronchodilation” and “chronic obstructive pulmonary disease triple therapy” with no date limits. Papers identified as described were searched manually. Distributed guidelines were GOLD 2014,10 GesEPOC 2014,2 NICE 20103 and The Canadian Thoracic Society recommendations for management of COPD 2007.4 Additional new evidence was distributed after the meeting, including articles identified through searches of the authors’ own files. Subsequent discussions by email led to the preparation of the first draft of this paper. On February 2015 the GI-DPOC convened again, and discussed the first draft of the paper in light of GOLD 2015.1 Additional new evidence was distributed after the meeting, including recent articles identified through searches of the authors’ own files. Subsequent discussions by email led to the preparation of the final text of this paper. The final reference list was generated on the basis of originality and relevance to the scope of this paper.
Should therapy be initiated early?A clinical diagnosis of COPD should be considered in any patient who has dyspnea, chronic cough or sputum production, and a history of exposure to risk factors for the disease. Spirometry is required to establish a diagnosis of COPD.1 The presence of a post-bronchodilator ratio of Forced Expiratory Volume in 1 second by Forced Vital Capacity (FEV1/FVC)<0.70 confirms the presence of persistent airflow limitation and thus of COPD and a normal value for spirometry effectively excludes the diagnosis of clinically relevant COPD.1 Spirometric screening of asymptomatic individuals is not supported by evidence. However, in individuals over 40 years old with a smoking history (>10 pack-years), spirometry may be performed with the aim of early diagnosis.5
A correct diagnosis is mandatory to decide the best treatment approach.11 Therapy should be initiated in early stages of the disease as even patients in GOLD stage 1 (as defined by GOLD prior to 2011) may respond to bronchodilators with physiological and symptomatic improvements.12,13 Also, several studies have reported improvement with bronchodilators in GOLD stage 2 patients (as defined by GOLD prior to 2011).14–16 A study assessing the ability of the GOLD 2011 stratification17 to predict the clinical course of COPD showed that group B had significantly poorer survival than group C, concluding that this group warrants special attention, requiring additional assessment and treatment.18
The Canadian Thoracic Society recommends therapy should be guided by the degree of disability, lung function and exacerbations4 whereas the National Institute for Health and Care Excellence (NICE) guidelines propose therapy based on a variety of parameters such as lung function, improvement in symptoms, activities of daily living, exercise capacity, rapidity of symptom relief, exacerbations, patient preference, side effects and cost.3 According to the GOLD guideliness, LAMA or LABA are alternative choices for group A patients and the recommended first choice for group B patients, with alternative choice of LAMA+LABA.1 However, dual bronchodilation with a fixed-dose combination of indacaterol/glycopyrronium (IND/GLY) has been reported to have clinically meaningful and symptomatic improvement in moderate to severe non-exacerbating COPD patients, who by definition fall within the B category.19,20 On the other hand, there are several potential advantages and beneficial effects of having a LABA+LAMA combination on the same device.21
The Spanish guidelines propose that COPD treatment should be based on four clinical phenotypes (non-exacerbator, exacerbator with emphysema, exacerbator with chronic bronchitis, mixed COPD-asthma) and disease severity, and recommend LABA+LAMA as an option for all phenotypes with severity level>II (on a scale of I to IV).2
Table 1 summarizes our proposals, suggestions and recommendations on diagnosis and early therapy.
Should therapy be initiated early?
| Diagnosis | • early diagnosis is of paramount importance to initiate treatment in the early stages of the disease |
| Therapy GOLD A patients | • monotherapy with a long-acting bronchodilator should be the first treatment choice |
| Therapy GOLD B patients | • in highly symptomatic GOLD B patients, dual bronchodilation should be initiated as soon as possible |
| Therapy in patients with bronchitic COPD | • the choice of single or dual bronchodilation will depend on the symptoms |
| • if the patient is a frequent exacerbator (≥2 exacerbations/year or 1 exacerbation requiring hospitalization), ICS should be added | |
| • in these patients, mucolytic agents are also drugs to consider |
ICS use has unequivocal benefits in asthma, but its position in COPD treatment is controversial.11 Different guidelines recommend the use of ICS or triple therapy in different patient categories given that patient stratification is not consensual across guidelines.1–4 The only apparent consensus is that ICS therapy alone is not recommended in COPD patients,1–5,22 and even in the treatment of exacerbations, the recommendation is to add oral or intravenous corticosteroids and/or antibiotics, depending on symptom severity and the existence of infection.1 Regardless of GOLD stage, severe exacerbations must be managed properly, since mortality increases as they become more frequent, particularly when these require hospital admission.23
Several recent studies have investigated the benefits of ICS on COPD,15,19–21,24–28 and the majority do not support the use of ICS in all patients. Two specific groups of patients may benefit from ICS: ACOS,7 with a reported prevalence ranging from 15% to 55%, depending on age and gender,29 whose patients cannot be treated with monotherapy LABA, and COPD with frequent exacerbations (≥2/year) regardless of treatment with bronchodilators.11 A very recent review of available studies strongly recommends limiting use of ICS to a minority of COPD patients who might benefit from it, and suggests some indicators of likelihood of ICS patients’ response.6
Several questions remain regarding the use of ICS in COPD patients: (a) do all ICSs increase the risk of pneumonia in COPD patients, or is it dependent on drug class?;11,12,15,25,30 (b) treatment with ICS for 3 years has been reported to be safe,15 but for more prolonged treatment the safety profile is still unknown;12 (c) is the use of a specific ICS and its dose relevant to the efficacy/safety ratio?;31 (d) Randomized Clinical Trials (RCTs) use very high doses of ICS – do they have the same safety and efficacy profile in lower doses?; and (e) should ICS be used only in patients for whom the benefits outweigh the risks?11 Risks associated to ICS use should be clearly established and balanced against its clinical benefits such as exacerbation control.
As for triple therapy, its recommendation in COPD patients at risk of exacerbations is generally accepted31–35 (one or more exacerbations per year, on average, for 2 consecutive years).35 Triple therapy seems to improve lung function, Quality of Life (QoL),28,33 and hospitalization rates,28 to provide greater reduction in airway wall thickness33 and seems to confer benefits on cardiovascular mortality.36 On the other hand, a National Health Service (NHS) review concluded that, although triple therapy decreased the hospitalization rate due to severe/acute COPD exacerbations compared with LAMA monotherapy, there was insufficient evidence to determine whether triple therapy was superior to dual bronchodilation.37 Several authors pointed out that evidence in favor of triple therapy use is scarce, study results heterogeneous,22,30,34 and the studies were too short to lead to any strong recommendations.34 However, it should be noted that future triple therapy, with all drugs in the same device, may have added advantages compared to triple therapy in different devices, given the possible synergism between molecules, as already shown for LABA+LAMA26 and ICS+LABA.38
Also, it has to be taken into account that triple therapy is expensive and has its own inherent risks.32 A recent review from UK general practice showed that, from 2004 to 2009, the use of triple therapy in all COPD degrees increased: from 25% to 59% in patients with very severe COPD, and as much as 14% and 19% in patients with mild and moderate COPD, respectively. Up to a third of patients with mild or moderate COPD were on triple therapy, and the authors raise the question of whether these patients are indeed frequent exacerbators, and thus are being treated according to current guidelines.39 These numbers are somewhat worrying given the available evidence, and may indicate that ICSs are being over-prescribed and, consequently, patients are being overtreated. Some authors propose that ICS should be withdrawn from patients who do not benefit from it. Gradual ICS withdrawal in moderate COPD patients does not increase moderate to severe exacerbations, although a greater decrease in lung function during the final step of withdrawal was shown.24
Some authors defend the position that, given the risks associated with ICS therapy, and the overall safety and effectiveness of long-acting bronchodilators, bronchodilators without ICS should be favored at all levels of COPD severity, except if there is an asthma component associated with COPD.6 Dual bronchodilation with IND/GLY may be preferable, since it has demonstrated superiority versus treatment with its monocomponents, indacaterol and glycopyrronium,26 suggesting the existence of synergistic activity. The potential advantages and beneficial effects of having a combination of LABA+LAMA on the same device are: (1) prevention of disease progression, (2) increased efficacy compared to single agents given their different mechanisms of action, (3) health status improvement by reducing symptoms and increasing physical activity, and (4) increased compliance – once daily, same device.21
GOLD recommends dual bronchodilation for symptom optimization as the second treatment choice in B, C and D patients.1
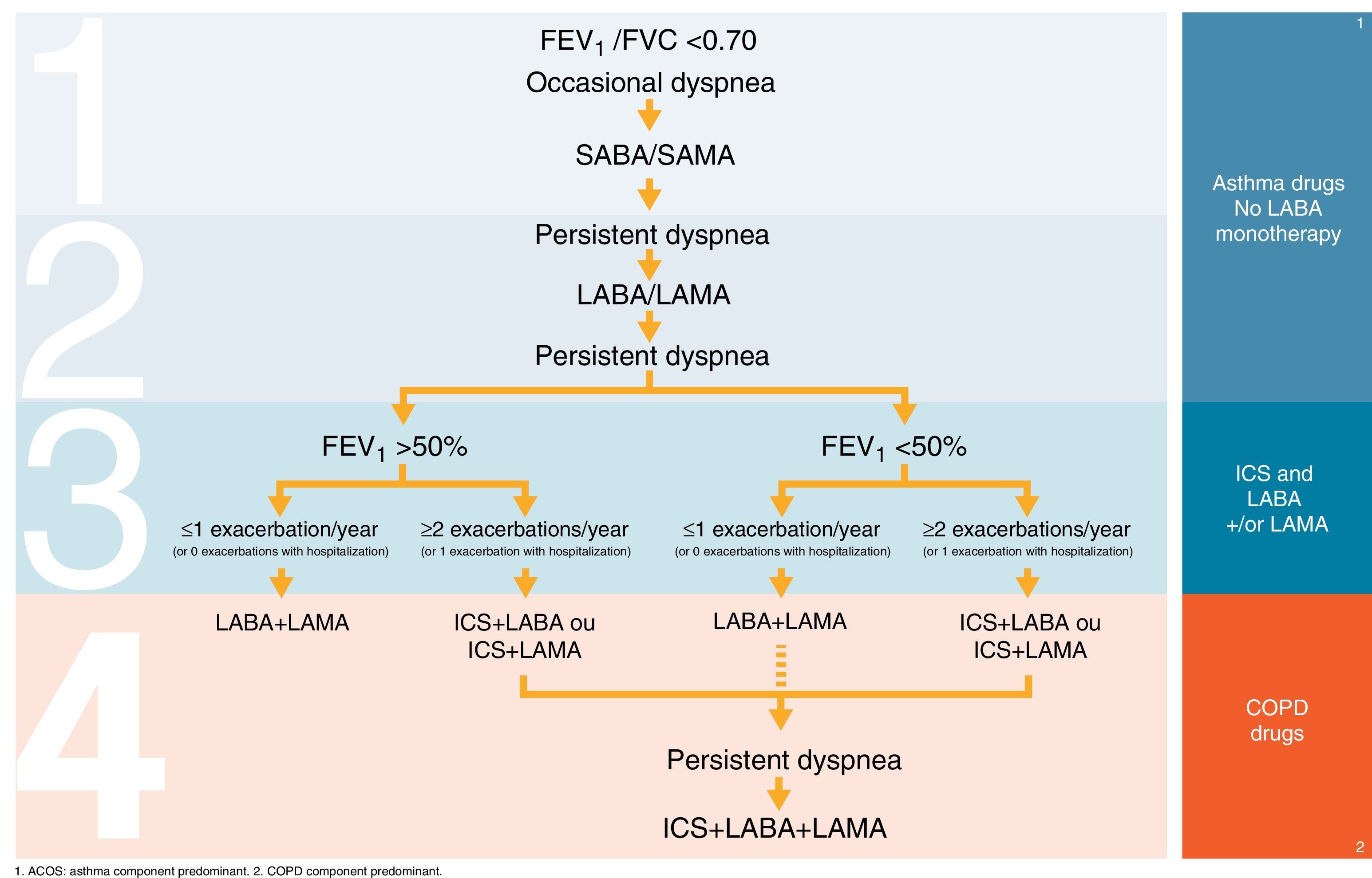
Table 2 and Fig. 1 summarize our proposals, suggestions and recommendations on COPD treatment sequence.
ICS and triple therapy.
| ICS and triple therapy | • proposal of therapeutic scheme depicted in Fig. 1 |
| Recommendation | • strong recommendation for a personalized, patient-oriented approach, taking into account each patient's clinical and personal characteristics and preferences |
Difficulties in early interventions (GOLD stage 1 and 2) are mainly due to low compliance, with increased mortality rate regardless of the type of therapy.12 A patient with dyspnea and fatigue will be more likely to comply with treatment whereas a less symptomatic patient will tend to be non-compliant. Focus should be given to informing patients12,19,20 about therapy benefits. As previously mentioned, one of the potential advantages and beneficial effects of having a combination LABA+LAMA therapy on the same device is an increased compliance – once daily.19–21 However, the choice of an inhaler should consider a range of different factors, including price and availability, similarity to other inhalers the patient uses, ability for the health care provider to correctly train the patient, ability of the patient to handle the inhaler, and patient preference.40 In this regard, studies have shown some advantages of the Breezhaler in comparison to the Handihaler in COPD patients with different disease severities: (1) Breezhaler has mechanisms that assure the drug is completely inhaled by the patient;41 (2) it delivers a higher fine particle fraction and generates a greater and more consistent intrathoracic deposition42 and (3) patients seem to prefer the Breezhaler because it is easier and more comfortable to use.41
As for the development of new drugs, the combination of a muscarinic antagonist with a beta2 agonist (MABA) is an interesting new approach, since it has the potential to demonstrate additive or synergistic bronchodilation over either component alone.43 A phase I trial to determine the pharmacokinetics of a MABA has already been completed, but the results are not yet available.44
Table 3 summarizes our proposals, suggestions and recommendations on compliance and new molecules.
Compliance and new molecules.
| Compliance | • proposal of focusing on patient information and education to increase compliance |
| • having a combination of drugs on the same device increases compliance, and the choice of an inhaler should be based on several factors | |
| New Molecules (MABA) | • results not available |
There are a few useful non-inhaled therapies that can be used in the management of COPD, namely mucolytic agents, macrolides, PDE4i, xanthine derivatives and immunostimulating agents. Some guidelines have already incorporated these additional therapies,1–3,45 but which therapy to use in which group of patients remains controversial.
Mucolytic agentsCarbocysteine, N-acetylcysteine (NAC) and erdosteine are the most common mucolytic agents used in COPD. They all exhibit antioxidant activity,1 with carbocysteine and NAC being also anti-inflammatory.46,47 Carbocysteine seems to decrease exacerbations in frequent exacerbators not receiving ICS.46,48 High-dose NAC seems to decrease exacerbation frequency regardless of ICS therapy,47,49 but only in patients at high risk of exacerbations.50 This effect appears to be particularly evident in patients with moderate COPD,51 but limitations due to study population may preclude generalization of these conclusions.52,53 Low-dose NAC has no effect on exacerbation frequency49,54,55 or clinical outcomes.54,55 Data from a meta-analysis supports the use of erdosteine in COPD, given the significant symptom improvement,56 but the reliability of this conclusion is disputed.57 The Spanish guidelines consider the addition of carbocysteine in the exacerbator patient with chronic bronchitis phenotype and severity level>II.2 GOLD considers carbocysteine as an option for GOLD D patients,1 and the NICE guidelines consider mucolytic therapy for patients with a chronic cough productive of sputum, but do not recommend routine use of mucolytic drugs to prevent exacerbations in patients with stable COPD.3
MacrolidesMacrolides are the most widely studied antibiotics for continuous long-term or intermittent use in COPD.58 Available evidence supports the continuous use of macrolides to prevent exacerbations59–62 and hospitalizations,62 but which patient population would benefit the most, as well as optimal dose and duration of treatment, remain unknown.58,62,63 A small study in patients with 3 or more exacerbations in the previous year showed that 500mg azithromycin 3 times per week for 12 months significantly decreased exacerbation rate and suggested that this regimen should be considered for use in patients with the frequent exacerbator phenotype who are refractory to standard care.60 However, a large study where 250mg azithromycin was given to patients daily for 12 months concluded that azithromycin was most effective in preventing exacerbations requiring both antibiotic and steroid treatment, and reported a greater efficacy in older patients and in those with less severe COPD.61 A systematic review including only trials with patients who were frequent exacerbators and who needed treatment with antibiotics or systemic steroids for those events, or who were on supplemental oxygen, concluded that the impact of intermittent use of macrolides (either 5 days every two months or 3 consecutive days per month) on exacerbation frequency and quality of life remains uncertain.59 A recent literature review concluded that, in Belgium, beyond the positive impact on exacerbations and related hospitalizations, the prevention of COPD exacerbations with azithromycin would not only be cost saving62 but also cost-effective.64,65
Two additional concerns regarding prophylactic long-term antibiotic treatment are the possibility of developing antibiotic resistance58,59,62,63,66 and the unknown potential adverse effects.58,59,63,66
All current guidelines recommend the use of antibiotics only for the treatment of infectious or severe exacerbations, and do not support the use of prophylactic antibiotic therapy.1–5 The Spanish guidelines also consider the addition of antibiotics in the exacerbator with chronic bronchitis phenotype with severity level IV.2 Macrolides are one of the first choice class of antibiotics to be used when necessary,1,3–5 but teophylline dose should be reduced during macrolide treatment3,5 since macrolides increase the serum concentration of theophylline.1
Phosphodiesterase-4 inhibitorsAlthough PDE4i are currently not available in Portugal, its use in COPD is already considered in the Spanish2 and GOLD1 guidelines. PDE4i are thought to reduce airway inflammation and bronchoconstriction. A very large systematic review concluded that PDE4i improve short-term lung function and reduce exacerbations, but have minimal or no impact on daily symptoms, QoL or life expectancy. Moreover, they are associated with gastrointestinal adverse effects and headaches, with roflumilast in particular being associated with significant weight loss and some psychiatric symptoms. These authors suggest that use of PDE4i should be limited to add-on therapy in a subgroup of patients with persistent symptoms or exacerbations despite optimal COPD management, and recognize that the role of PDE4i and its positioning in COPD management remains to be defined.67 Another very large systematic review of roflumilast reached the same overall conclusions, and further reported that roflumilast significantly reduced moderate to severe exacerbations, but not severe exacerbations alone.68 Both reviews agree that longer-term trials are needed to determine the actual benefits of PDE4i and their long-term safety. A more recent study confirmed these results by reporting a borderline statistical significance in the reduction of severe COPD exacerbations with roflumilast versus placebo in patients with severe to very severe COPD.69 However, all roflumilast results derived from industry-sponsored trials have been disputed, with some authors defending that these trials were appropriate70 and others criticizing them.71 A very recent study concluded that, in patients with severe chronic obstructive pulmonary disease at risk for exacerbations, already receiving ICS+LABA, with or without tiotropium, roflumilast reduces exacerbations and hospital admissions,72 and these results were corroborated in a small “real-life” population of severe COPD patients with frequent exacerbations already receiving triple therapy.73 Roflumilast showed particular benefit in patients with 4 or more exacerbations prior to initiating therapy.73
The Spanish guidelines consider the addition of PDE4i in the ACOS phenotype with severity level IV if associated with exacerbations and sputum, and in the exacerbator with chronic bronchitis phenotype with severity level>I PDE4i are one of the recommended choices.2 GOLD guidelines recommend PDE4i as alternative choice treatments for GOLD C and D patients, and state that they may be useful to reduce exacerbations in patients with FEV1<50% predicted, chronic bronchitis, and frequent exacerbations. Despite this, PDE4i should always be used in combination with at least one long-acting bronchodilator.1
MethylxanthinesMethylxanthines are nonspecific inhibitors of all phosphodiesterase enzyme subsets, and have a wide range of adverse effects1 related to plasma concentrations, including nausea, vomiting, and headaches and, at higher concentrations, cardiac arrhythmias and seizures.74 Theophylline, the most widely used methylxanthine, has shown clinically relevant results in patients with stable COPD, namely FEV1 and FVC improvement,75,76 as well as improvements in maximal O2 uptake (VO2max), O2 partial pressure (PaO2) and CO2 partial pressure (PaCO2).76 Theophylline has a role in the management of stable COPD, and is currently used as add-on therapy in severe COPD patients not controlled by bronchodilator therapy.74 However, its wider use is hampered by the fact that blood levels need to be closely monitored due to serious, even potentially life-threatening side-effects.75,76 As for the use of methylxanthines in the management of COPD exacerbations, current evidence does not support it, given that the possible beneficial effects in lung function and clinical endpoints are modest and inconsistent, whereas adverse effects are significant.77 All current guidelines stand by this point of view.
The Spanish guidelines consider the addition of theophylline in all phenotypes, but only with severity level IV.2 GOLD recommends theophylline as an alternative choice treatment for all GOLD classes, but only if other long-term treatment bronchodilators are unavailable or unaffordable.1 The NICE guidelines propose that theophylline should only be used after a trial of short-acting bronchodilators and long-acting bronchodilators, or in patients who are unable to use inhaled therapy.3 The Canadian guidelines only recommend trying theophylline in patients with severe symptoms and frequent exacerbations despite use of both tiotropium and a LABA/ICS.4 In the management of exacerbations, intravenous methylxanthines (theophylline or aminophylline) are considered second-line therapy, only to be used in selected cases when there is insufficient response to short-acting bronchodilators.1,3,5 When using theophylline, it is necessary to monitor blood levels, side effects and potential drug interactions.4
Immunostimulating agentsA few small studies have reported a potential benefit of the immunostimulating agent OM-85 in the prevention of severe respiratory events, risk of hospitalization and duration of hospital stay in severe and very severe COPD patients,78 and in the decrease of acute exacerbations, symptoms, and need for antibiotics and symptomatic relief medications, in patients with chronic bronchitis and COPD.79 OM-85 was well tolerated.79 In patients with chronic bronchitis or mild COPD, a decrease in exacerbation frequency was observed after 6 months of intermittent OM-85 administration (30 days followed by three 10-day courses for months 3, 4 and 5), and OM-85 was also well tolerated.80 However, these studies were small and the longest follow-up period lasted for only 1 year.
GOLD does not recommend regular use of immunostimulating agents due to the lack of studies on their long-term effects.1
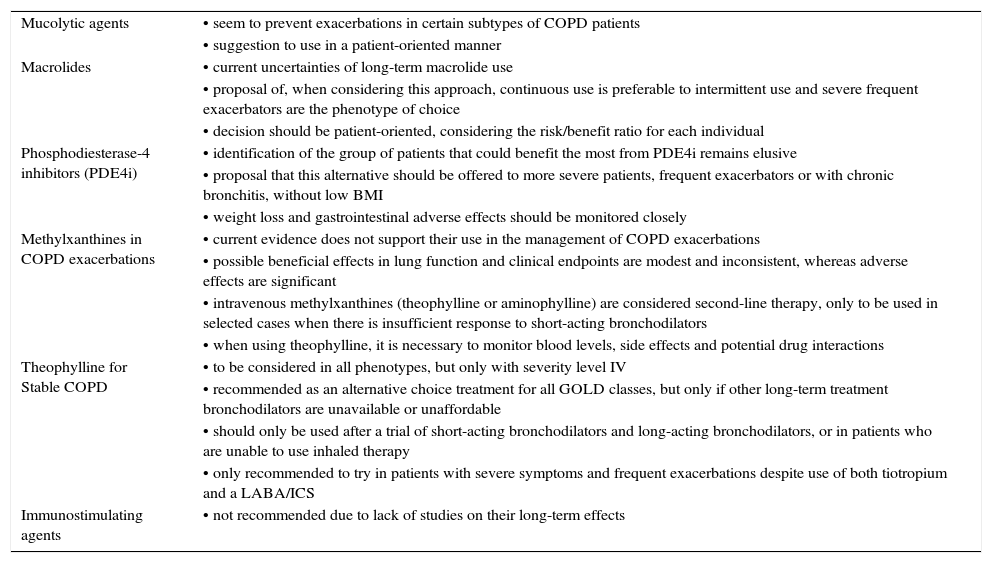
Table 4 summarizes our proposals, suggestions and recommendations on additional non-inhaled therapies.
Additional useful non-inhaled therapies.
| Mucolytic agents | • seem to prevent exacerbations in certain subtypes of COPD patients |
| • suggestion to use in a patient-oriented manner | |
| Macrolides | • current uncertainties of long-term macrolide use |
| • proposal of, when considering this approach, continuous use is preferable to intermittent use and severe frequent exacerbators are the phenotype of choice | |
| • decision should be patient-oriented, considering the risk/benefit ratio for each individual | |
| Phosphodiesterase-4 inhibitors (PDE4i) | • identification of the group of patients that could benefit the most from PDE4i remains elusive |
| • proposal that this alternative should be offered to more severe patients, frequent exacerbators or with chronic bronchitis, without low BMI | |
| • weight loss and gastrointestinal adverse effects should be monitored closely | |
| Methylxanthines in COPD exacerbations | • current evidence does not support their use in the management of COPD exacerbations |
| • possible beneficial effects in lung function and clinical endpoints are modest and inconsistent, whereas adverse effects are significant | |
| • intravenous methylxanthines (theophylline or aminophylline) are considered second-line therapy, only to be used in selected cases when there is insufficient response to short-acting bronchodilators | |
| • when using theophylline, it is necessary to monitor blood levels, side effects and potential drug interactions | |
| Theophylline for Stable COPD | • to be considered in all phenotypes, but only with severity level IV |
| • recommended as an alternative choice treatment for all GOLD classes, but only if other long-term treatment bronchodilators are unavailable or unaffordable | |
| • should only be used after a trial of short-acting bronchodilators and long-acting bronchodilators, or in patients who are unable to use inhaled therapy | |
| • only recommended to try in patients with severe symptoms and frequent exacerbations despite use of both tiotropium and a LABA/ICS | |
| Immunostimulating agents | • not recommended due to lack of studies on their long-term effects |
There are no conclusive data establishing that treatment of COPD co-morbidities will reduce morbidity and mortality rates in patients. The fact that patients with clinically relevant co-morbidities are usually excluded from clinical trials of COPD, and that patients with COPD are also usually excluded from clinical trials on other pathologies, has prevented evidence-based clinical treatment guidelines in these patients. However, co-morbidities should be adequately controlled due to their potential negative impact on COPD prognosis.1
Cardiovascular co-morbiditiesCardiovascular medicationsA large retrospective study reported that statins, angiotensin-converting-enzyme inhibitors (ACEi), and angiotensin-receptor blockers (ARBs) could have cardioprotective properties in patients with COPD, substantially altering their prognosis. Also, COPD hospitalization and total mortality rates were reduced, as well as the myocardial infarction rate in COPD patients with high cardiovascular risk, independently of the concomitant use of steroids. The largest benefits were observed with the combination of statins and either ACEi or ARBs.81 Several further studies were conducted to assess the potential effect of statins and ACEi on COPD, and reviews of those studies concluded that they were beneficial.82–86 However, all these studies were observational, and a very recent large RCT on the effects of simvastatin on exacerbations and FEV1 in COPD patients was terminated due to futility.87 Other RCTs also failed to prove the benefits of ACEi88 or ARBs89 in different COPD outcomes. GOLD guidelines do not mention the use of these medications and state that cardiovascular co-morbidities should be treated according to their specific guidelines.1
β-BlockersThere has been concern that prescription of β-blockers for patients with COPD might cause bronchoconstriction and worsen respiratory symptoms.12 However, cardioselective β-blockers have been shown to be associated with reduced mortality in patients with COPD undergoing vascular surgery,90 and a meta-analysis did not find significant differences in FEV1 or respiratory symptoms between patients treated with a cardioselective β-blocker and those treated with placebo, even in patients with severe COPD.91 Another very recent meta-analysis concluded that the use of β-blockers in patients with COPD with or without co-morbid cardiovascular diseases, may not only decrease the risk of overall mortality but also reduce the risk of exacerbations, concluding that, given the benefits of β-blockers in conditions such as hypertension, heart failure and coronary artery disease, these drugs should not be withheld because of COPD.92 In a fairly large population (n=825) of inpatients admitted for acute exacerbations of COPD, the use of β-blockers was well tolerated and associated with reduced mortality.93 Current guidelines recommend the use of β-blockers to treat specific associated co-morbidities,1,2 preferably cardioselective β-blockers,1 with the added advantage of reducing exacerbations and mortality associated with some cardiovascular co-morbidities.2
Gastroesophageal reflux disease (GERD)GERD is one of the most common causes of chronic cough and is a frequent co-morbidity in COPD patients, also being associated with exacerbations.94–96 Older age and female gender95 are associated with GERD in COPD patients, and the association between GERD and obesity is well known. A very recent large study reported that COPD patients with GERD were at increased risk of medically treated COPD exacerbations, defined as a short course treatment with oral corticosteroids alone or in combination with antibiotics, only if they did not use acid inhibitory treatment regularly.96 These results are in accordance with a small study that reported that proton pump inhibitor use was associated with a significant decrease in COPD exacerbations in older patients.97 Also, it has been reported that most of COPD medications except LAMA are associated with GERD.95 GOLD guidelines state that proton pump inhibitors are often used for treatment of GERD, but the most effective treatment for this condition in patients with COPD has yet to be established.1
Table 5 summarizes our proposals, suggestions and recommendations on the treatment of cardiovascular co-morbidities and GERD.
Treatment of co-morbidities.
| Cardiovascular | • cardiovascular co-morbidities should be treated according to their specific guidelines |
| • in the presence of some cardiovascular co-morbidities, cardioselective beta-blockers are not only safe but also beneficial in the control of COPD | |
| Gastroesophageal reflux disease (GERD) | • special attention should be given to older patients, women, and obese patients |
| • a proton pump inhibitor is advisable |
Although this paper proposes a therapeutic scheme for COPD patients, a full consensus on optimal treatment sequence in COPD cannot be found at present. There are still too many unknown variables, no reliable biomarkers to guide treatment, and a poor definition of clinically relevant COPD phenotypes. It is imperative that active research continues in order not only to identify specific groups of patients that can benefit from specific treatments, but also to develop new molecules or combinations of molecules, that are more effective in symptom control. The ultimate goal would be to stop disease progression, but this goal may not be attainable. At present, COPD should be treated in a personalized, tailor-made, patient-oriented manner.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare collaborating and receiving fees from pharmaceutical companies other than Novartis either through participation in advisory board or consultancy meetings, congress symposia, clinical trial conduct or investigator-initiated trials.
Role of funding sourceFunding for this paper was provided by Novartis Portugal. Funding was used to access all necessary scientific bibliography and cover meeting expenses. Novartis Portugal had no role in the collection, analysis and interpretation of data, in the writing of the paper and in the decision to submit the paper for publication.
The authors wish to thank Novartis Portugal for the funding for this paper, which was used to access all necessary scientific bibliography and cover meeting expenses.