Evidence supporting the utilization of surface EMG (sEMG) of extra-diaphragmatic muscles for monitoring of mechanical ventilation (MV) assistance is unclear. The purpose of this review was to assess the quality of literature available on using extra-diaphragmatic sEMG as an assessment technique of respiratory responses during MV.
MethodsStudies using sEMG of extra-diaphragmatic respiratory muscles during MV were selected by two independent researchers after performing a database search of PubMed, CINAHL, GOOGLE SCHOLAR. Exclusion criteria were studies of patients with neuromuscular disorders, receiving neuromuscular blocking agents, receiving non-invasive MV, using needle EMG, and studies written in languages other than English. Quality of identified studies was assessed with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2). This study is registered with PROSPERO, number (CRD42018081341).
Results596 references were identified. Of the identified studies, 7 studies were included in the review. Findings demonstrate that sEMG of extra-diaphragmatic muscle activity is a valid and applicable tool to evaluate mechanical loading/unloading of respiratory muscles and respiratory drive or sensation. However, the quality of literature supporting sEMG as monitoring tool of respiratory responses were characterized by a high and unclear risk of bias.
ConclusionsAlthough it appears to be a valid and applicable tool, there is a scarcity of literature that directly demonstrates the diagnostic accuracy of sEMG of extra-diaphragmatic muscles in monitoring respiratory mechanics and respiratory drive or sensation during MV assistance across wide populations and conditions.
Although mechanical ventilation (MV) is an essential life-saving therapy, it may result in a rapid diaphragmatic weakness due to its reduced activity.1 Diaphragmatic disuse atrophy and protein proteolysis can occur rapidly during MV, resulting in a progressive diaphragmatic dysfunction.1,2 Hence, tailoring MV assistance to the patients’ needs is an important objective to maintain adequate diaphragmatic function and, thus, accelerating the MV liberation process.3,4 This objective increases the need for rigorous assessment of patient-ventilator interaction.5
Increased work of breathing (WOB) is manifested by the use of respiratory extra-diaphragmatic muscles (e.g., external intercostal, parasternal intercostal, sternocleidomastoid, scalene) to compensate for the overload imposed on diaphragmatic capacity.6–10 This sign becomes prominent as neuro-respiratory drive (NRD) increases in patients during MV liberation failure.10–12 In other words, the increased activation of respiratory extra-diaphragmatic muscles is an attempt to achieve balance between mechanical load (demand) and respiratory muscle capacity (supply).13
NRD can be evaluated invasively via an esophageal EMG catheter placed at the level of the diaphragm14; this technique is associated with technical complexity and potential risks.15,16 In contrast, the use of surface EMG (sEMG) of extra-diaphragmatic muscles is another method for assessing NRD which has been introduced as a promising assessment tool to evaluate respiratory loading/unloading and respiratory sensation during MV.17–19
The rationale behind using sEMG stems from its non-invasiveness and easy practical use.20,21 These advantages are highlighted, as patients in the intensive care unit (ICU) are vulnerable to infections and at an increased risk of complications.16,22 In addition, the activation of extra-diaphragmatic muscles when ventilatory demand outweighs ventilatory capacity makes these muscles a practical choice for the detection of increased loading and neural drive.17 Also, the diaphragm moves significantly during inspiration, making it difficult to obtain accurate EMG signal from surface electrodes, whereas this is not an issue for extra-diaphragmatic muscles.23
Although sEMG has been used to detect clinical deterioration, inspiratory muscle fatigue, and respiratory muscle endurance,24,25 the quality of evidence supporting its utilization with extra-diaphragmatic muscles during MV assistance is unclear. Therefore, the aim of this non-comparative systematic review is to assess the evidence supporting the use of extra-diaphragmatic sEMG to evaluate respiratory responses during MV assistance.
MethodsGiven the novelty of using sEMG to assess respiratory mechanics with patients receiving MV, the database search was performed with no date restrictions (last search was completed October 2019). This review was prospectively registered with PROSPERO (CRD42018081341). A medical librarian was consulted and helped select appropriate databases and search terms. A comprehensive search was performed using the following databases: PubMed, CINAHL, and GOOGLE SCHOLAR. For example, in PubMed, the search strategy resulted in the following string: “(respiratory muscles OR inspiratory muscles OR accessory muscles OR scalene OR sternocleidomastoid OR parasternal OR intercostal) AND (electromyography OR EMG) AND (mechanical ventilation OR artificial respiration) AND (muscle activity OR respiratory mechanics OR evaluation OR assessment).” Medical Subject Headings (MeSH) were used to facilitate records identification. The same search terms were used in all databases, respecting the differences in search strategy for each one.
The inclusion criteria were (1) studies using sEMG (2) of extra-diaphragmatic muscles (3) during invasive MV, (4) in adolescent and adult patients (≥13 years old). Studies were excluded based on the following exclusion criteria: (1) patients with neuromuscular disorders, (2) patients receiving neuromuscular blocking agents, (3) use of needle EMG, and (4) studies written in languages other than English.
Following the comprehensive search of databases, two independent reviewers screened titles and abstracts according to the inclusion criteria. Then, the selected studies went through full text screening to determine their eligibility for the review and therefore, data extraction. The studies were selected based on the consensus of the two reviewers (HYA and JDL); there were no disagreements necessitating a third reviewer. The main results of each of the selected articles were summarized and tabulated. Methodological quality of selected studies was assessed with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool, which is recommended for use in systematic reviews by Cochrane Collaboration.22
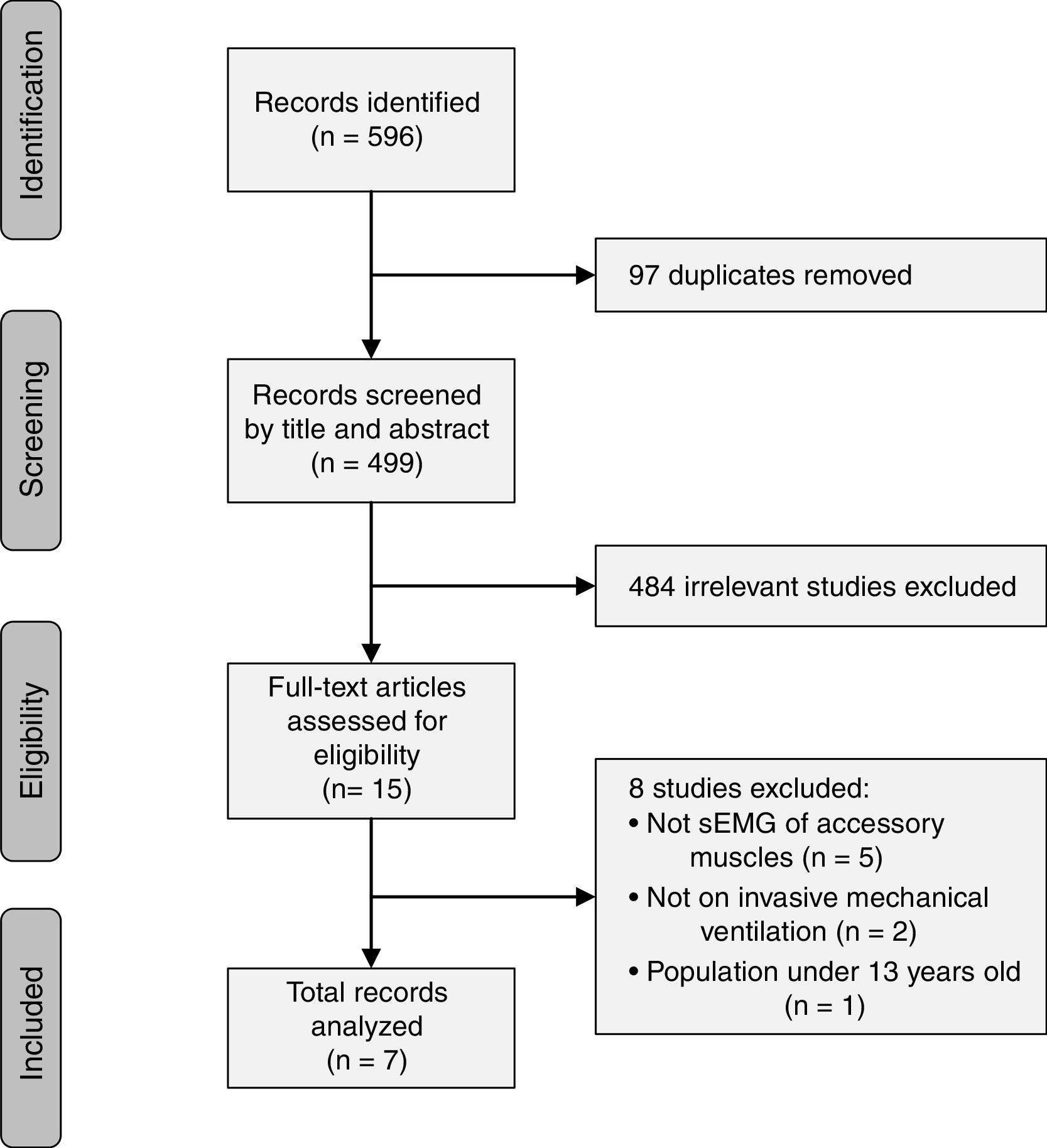
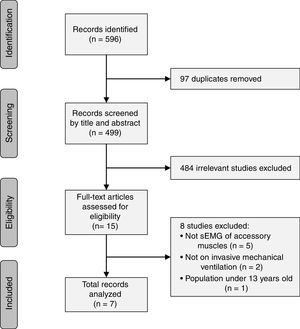
ResultsThe literature search yielded a total of 596 studies which were identified based on their titles and abstracts. The final included studies were defined by consensus of the two reviewers, and 7 studies were included in the present review (Fig. 1). The 7 studies were written by 6 different author groups. A single group of 3 researchers collaborated on 2 different studies that met the inclusion criteria of this review.17,26 Three of the studies were conducted in France, 2 studies in Switzerland, 1 study in Germany, and 1 in Italy.
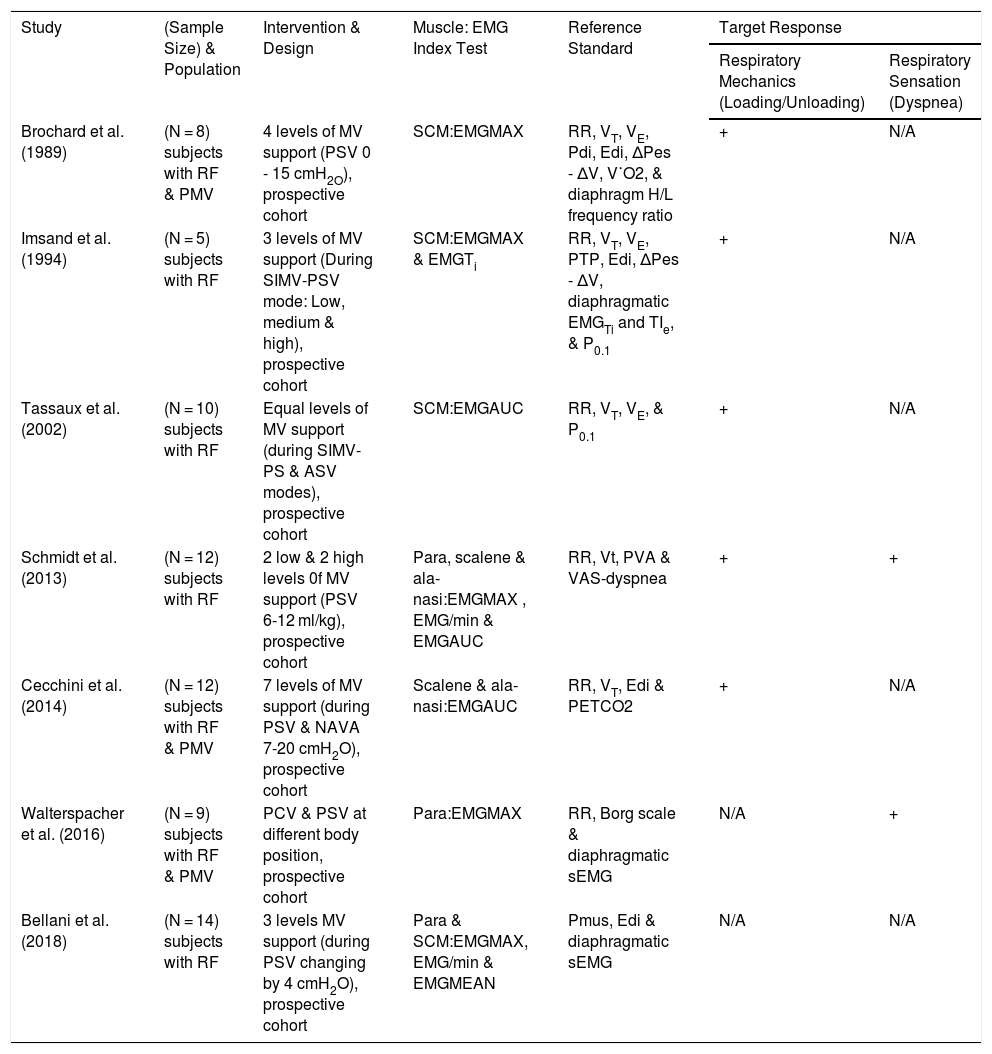
Heterogeneity of the methods used in the included studies and results presented precluded our planned meta-analysis. Hence, we described the studies qualitatively according to the first author and publication year, sample size, study design and population, type of intervention or MV settings, index tests, reference tests, and target conditions or responses. A summary of the results is described in the Table 1 provided following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy (PRISMA-DTA).27
Overview of sEMG Index Tests and Its Assessment Ability of Respiratory Mechanics and Respiratory Sensation.
| Study | (Sample Size) & Population | Intervention & Design | Muscle: EMG Index Test | Reference Standard | Target Response | |
|---|---|---|---|---|---|---|
| Respiratory Mechanics (Loading/Unloading) | Respiratory Sensation (Dyspnea) | |||||
| Brochard et al. (1989) | (N = 8) subjects with RF & PMV | 4 levels of MV support (PSV 0 - 15 cmH2O), prospective cohort | SCM:EMGMAX | RR, VT, VE, Pdi, Edi, ΔPes - ΔV, V˙O2, & diaphragm H/L frequency ratio | + | N/A |
| Imsand et al. (1994) | (N = 5) subjects with RF | 3 levels of MV support (During SIMV-PSV mode: Low, medium & high), prospective cohort | SCM:EMGMAX & EMGTi | RR, VT, VE, PTP, Edi, ΔPes - ΔV, diaphragmatic EMGTi and TIe, & P0.1 | + | N/A |
| Tassaux et al. (2002) | (N = 10) subjects with RF | Equal levels of MV support (during SIMV-PS & ASV modes), prospective cohort | SCM:EMGAUC | RR, VT, VE, & P0.1 | + | N/A |
| Schmidt et al. (2013) | (N = 12) subjects with RF | 2 low & 2 high levels 0f MV support (PSV 6-12 ml/kg), prospective cohort | Para, scalene & ala-nasi:EMGMAX , EMG/min & EMGAUC | RR, Vt, PVA & VAS-dyspnea | + | + |
| Cecchini et al. (2014) | (N = 12) subjects with RF & PMV | 7 levels of MV support (during PSV & NAVA 7-20 cmH2O), prospective cohort | Scalene & ala-nasi:EMGAUC | RR, VT, Edi & PETCO2 | + | N/A |
| Walterspacher et al. (2016) | (N = 9) subjects with RF & PMV | PCV & PSV at different body position, prospective cohort | Para:EMGMAX | RR, Borg scale & diaphragmatic sEMG | N/A | + |
| Bellani et al. (2018) | (N = 14) subjects with RF | 3 levels MV support (during PSV changing by 4 cmH2O), prospective cohort | Para & SCM:EMGMAX, EMG/min & EMGMEAN | Pmus, Edi & diaphragmatic sEMG | N/A | N/A |
N/A, data not available, + responsive; PSV, pressure support ventilation; SIMV, synchronized intermittent mandatory ventilation; ASV, adaptive synchronized ventilation; NAVA, neurally adjusted ventilatory assist; PCV, pressure control ventilation; COPD, chronic obstructive pulmonary disease; RF, respiratory failure; PMV, prolonged mechanical ventilation; SCM, sternocleidomastoid; para, parasternal; EMGMAX, maximum EMG activity; EMGAUC, EMG area under the curve; EMG/min, EMG activity per minute; EMGMIN, minimum EMG activity; EMGMEAN, mean EMG activity; EMGMAX-MIN, maximum-minimum EMG activity; Pdi, trans-diaphragmatic pressure; Edi, electrical activity of the diaphragm; RR, respiratory rate; VT, tidal volume; V˙E,minute ventilation; V˙O2, oxygen uptake; P0.1, occlusion pressure at 1 milliseconds; PTP, pressure time product; ΔPes –ΔV, esophageal pressure – volume curve; EMGTi, duration of electrical activity; TIe mechanical duration of inspiratory effort; H/L, high/low; VAS, visual analogue scale; PVA, patient-ventilator asynchrony; Pmus, inspiratory muscle pressure; PETCO2, end tidal carbon dioxide.
Maximum EMG activity (EMGMAX or EMGMAX%) was a commonly used parameter to assess muscle activity (n = 5); this value represents EMG activity relative to the peak EMG signal obtained during any inspiratory effort.17,28–31 EMG area under the curve (EMGAUC) was used in 3 studies17,26,32; this is the mathematical integral of the absolute value of raw EMG signals, expressed as a proportion of the maximum value. Duration of EMG activation (EMGTi) was reported in 1 study for SCM.30 EMG activity per minute (EMG/min), was calculated as EMGAUC x (total respiratory rate).17 Mean EMG activity (EMGMEAN; average EMG activity during 40 respiratory cycles), and EMGMAX-EMGMIN (EMGMAX-MIN; during 40 respiratory cycles) were reported in 1 study.28
Extra-diaphragmatic muscles tested were: sternocleidomastoid (SCM) (n = 4 studies),28,30–32 parasternal (n = 3),17,28,29 scalene (n = 2),17,26 and ala-nasi (n = 2).17,26 Of the included studies, diaphragmatic activity was assessed via invsavie espohageal catheter (Edi) in 4 studies26,28,30,31 and via sEMG in 2 studies.28,29
All of the included studies were prospective and used pressure support ventilation (PSV) as a spontaneous mode of MV for weaning, or incorporated PSV with the use of synchronized intermittent mandatory ventilation (SIMV; n = 2),30,32 adaptive support ventilation (ASV; n = 1),32 neurally adjusted ventilatory assist (NAVA; n = 1).26 Study populations mainly included subjects with respiratory failure due to various cardiopulmonary conditions and/or prolonged MV.
sEMG of extra-diaphragmatic muscles and respiratory conditionsAll studies used sEMG to detect respiratory muscle responsiveness to varying levels of MV support, modes, or body positions. We found that sEMG of extra-diaphragmatic muscles was used as a surrogate tool to monitor two main respiratory responses or target conditions: mechanical loading/unloading of respiratory muscles and respiratory sensation or dyspnea.
Data showed that extra-diaphragmatic muscle activity was responsive to mechanical loading/unloading and/or respiratory sensation during MV assistance (Table 1). Physiological measurements referenced to the response of sEMG activity that may reflect mechanical loading/unloading of respiratory muscles include: respiratory rate (RR), tidal volume (VT), minute ventilation (V˙E), oxygen consumption (V˙O2), WOB (quantified by esophageal pressure (Pes) plotted against volume (V) during active and passive breathing), end-tidal CO2 (PETCO2), diaphragmatic activity measured via Edi, diaphragmatic activity measured with sEMG, high/low diaphragmatic EMG frequency ratio (to detect diaphragmatic fatigue), pressure-time product (PTP) (calculated by (Pes) plotted against Time (T)), and trans-diaphragmatic pressure (Pdi) (calculated by gastric pressure – Pes), mechanical duration of inspiratory effort (TIe) and EMG duration (EMGTi) of the diaphragm. Physiological measurements referenced to sEMG in monitoring respiratory sensation include: visual analog scale (VAS) and Borg scale.
Mechanical loading/unloading of respiratory musclesWe evaluated the response of sEMG activity in each study and determined its responsiveness to of MV assistance based on changing ventilatory, mechanical, and neural output (increase, decrease, or no change). Of note, most of the studies did not report statistical correlation between sEMG and reference tests.
Activity of SCM,31,32 parasternal,17 scalene,17,26 and ala-nasi,17,26 muscles were responsive to the level of MV assistance reflected by the loading/unloading of respiratory muscles and its effect on RR, VT,17,26,31,32V˙E, WOB (ΔPes -ΔV),31 Pdi, Edi, H/L ratio of diaphragmatic EMG activity,31PETCO2,26 P0.1,32 and the prevalence of ineffective triggering effort.17 One study showed that sEMG activity of extra-diaphragmatic muscles was directly correlated with Edi (r = 0.49 to 0.71, p < 0.0001).26
SCM activity was similar between spontaneous and mandatory breaths during SIMV + PSV mode in one study, which was validated by constant WOB, V˙E, PTP and TIe, P0.1, EMGTi.30 In contrast, while SCM activity matched constant ventilatory output during spontaneous and controlled breaths, its response was slow to the changes in ventilatory output across MV levels of assistance during PSV + SIMV mode.30 However, the aggregate value for both SCM and Edi activity was responsive to change in WOB during high levels of MV assistance (sEMG activity decreased as MV support increased).30
Respiratory sensationOnly two of the seven included studies assessed respiratory sensation in which extra-diaphragmatic muscle activity was responsive to the level of dyspnea.17,29 In one study, dyspnea was evaluated using VAS, which was directly correlated with EMGAUC and EMGMAX of parasternal, ala-nasi, and scalene muscles activity (r = 0.72 to 0.98, p < 0.0001).5 The other study found that neither parasternal activity nor dyspnea level, measured using Borg scale changed with varying body positions, indicating a matched response between dyspnea and parasternal EMG measurements.29
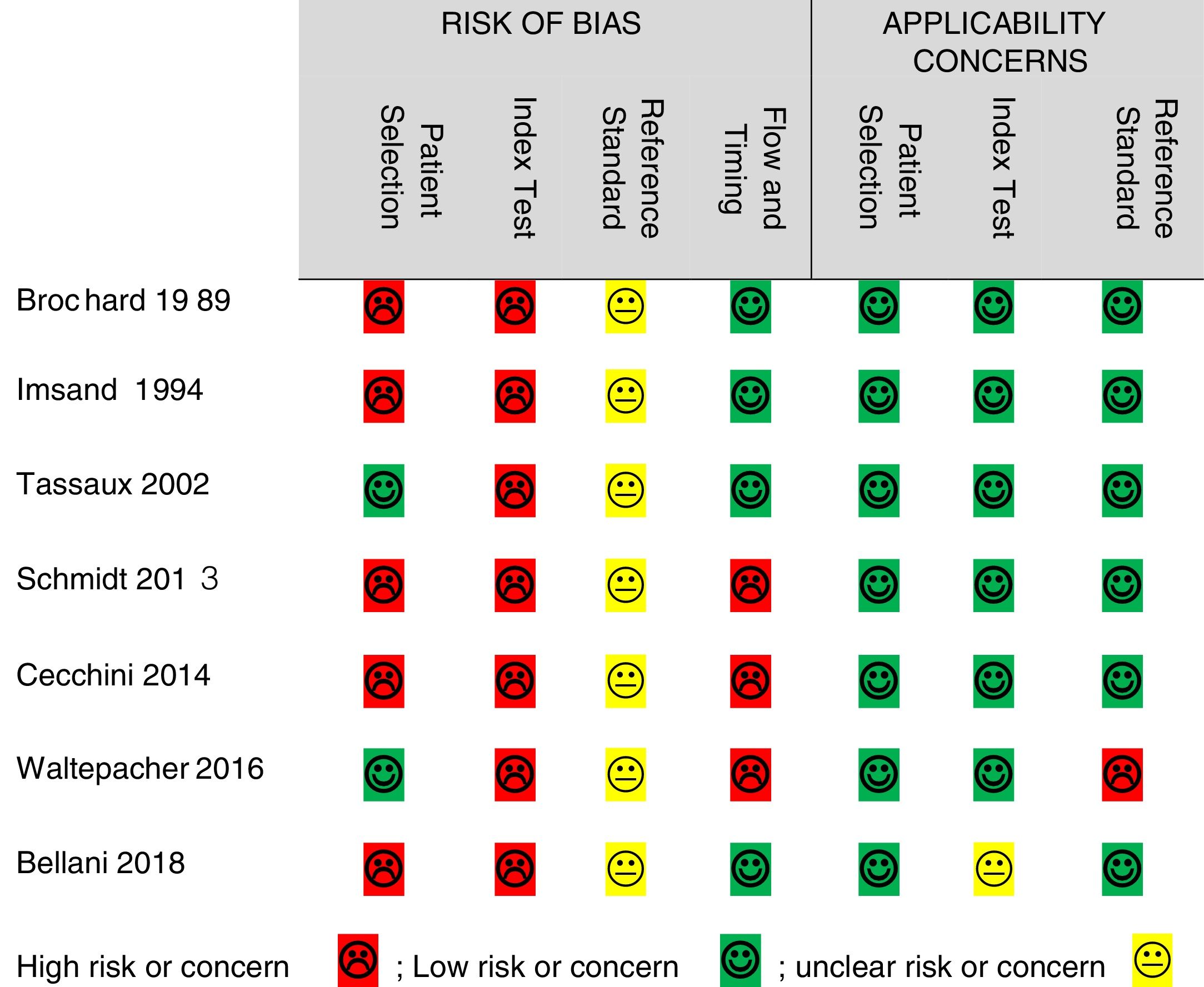
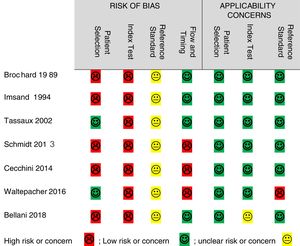
Quality assessmentThe QUADAS-2 tool was used to assess the quality of the included studies in terms of risk of bias and applicability concerns. Risk of bias was high in all 7 studies for the “index test,” 5 studies for “patient selection,” 3 for “flow and timing,” and the risk was unclear in all studies for the “reference standard.” Regarding applicability concerns, 1 study had unclear risk for the “index test”, 1 study had high concern for the “reference standard”, and all studies had low concern for “patient selection” (Fig. 2).
DiscussionThis review was conducted to evaluate the quality of evidence available on using sEMG of extra-diaphragmatic muscles to assess respiratory responses during MV assistance. Data show that sEMG of extra-diaphragmatic muscles is regarded as a valid and applicable tool for assessing changes in mechanical loading/unloading of respiratory muscles and respiratory sensation during MV assistance. We found that the response of extra-diaphragmatic muscle activity measured via sEMG matched at least one of the respiratory responses summarized in this review (i.e., respiratory mechanical loading/unloading or respiratory sensation) in all studies except for one study, which did not report sufficient data of extra-diaphragmatic muscles responses in their results.28 However, the included studies lack the evidence of sEMG accuracy in assessing MV clinical outcomes (i.e., respiratory failure, MV liberation readiness, or success/failure). This is partly because these studies did not primarily use sEMG to determine its diagnostic accuracy and only used it as a research tool to show the effectiveness of various MV interventions.
The matching response of extra-diaphragmatic sEMG activity with the ventilatory output is due to the activation of these muscles as a compensatory response for increased ventilatory load and NRD, which are associated with weak diaphragm or under-assistance in patients with MV.11,17,31 During the early phase of a failed MV liberation trial, patients display an increased mechanical load compared with those who had successful trial.10 Extra-diaphragmatic muscle activity is increased to offset the declining ventilatory function,11 which can explain the strong relationship between dyspnea and EMG activity of extra-diaphragmatic muscles in one of the included studies in this review.17
In light of this review’s findings, the response of sEMG of extra-diaphragmatic muscle activation to MV assistance should be interpreted with caution. The mode of MV can be a confounding factor that interferes with the mechanical or neural output. For example, in one study the use of V˙E was a weak parameter as a reference standard during ASV mode because it is pre-set to deliver a constant level of MV assistance.32 Similarly, inspiratory neuromuscular output is pre-programmed for a given level of assistance and not based on a breath-by-breath basis during SIMV mode.30 This explains the slow response of ventilatory parameters and SCM re-programming after a sustained change in ventilatory load in one study.30 In addition, ventilatory compensation can be achieved by both RR and VT to reach a constant V˙E with the changing level of MV assistance.17,30,32 One study reported an increase in RR but did not report VT,29 giving an incomplete picture of ventilatory output, since it could be interpreted as shallow breathing and not increased ventilatory efficiency as the study implies. Hence, extra-diaphragmatic sEMG activity should be considered with the reporting of both RR and VT collectively as reference standards. Finally, posture should be taken into account as it can influence respiratory muscle activation.33 Sitting position compared to supine and semi-recumbent positions reduced NRD to the diaphragm and not the parasternal muscle or dyspnea level during MV liberation trial.29 Similarly, sitting position was found to require less activation of diaphragm and intercostal muscles compared to supine position with no effect on ventilatory output.34
The high risk of bias reported for patient selection is mainly related to the design of the studies in which random sampling was not performed or not reported (n = 4)17,26,30,31 and for the exclusion of patients with COPD (n = 1).28 The high risk of bias found in all of the studies for the “index test” is due to the fact that these studies did not use blinding of the researcher to the reference standards. This means that sEMG was interpreted with the prior knowledge of the reference standard results, which mainly represent instantaneous changes in ventilatory output during MV. Likewise, not blinding the researcher to the index test affected the quality of evidence for the “reference standards.” However, sEMG results often take time for processing which makes them less likely to generate bias in the instant results of the reference standards (ventilatory output). Hence, in addition to the lack of reporting of blinding, we evaluated the risk of bias for the reference standards to be unclear in all the studies.
High risk of bias for flow and timing in three studies was due to the exclusion of a total of 4 patients from the analysis in two studies due to the inability to record scalene sEMG17,26 and one patient for the inability of measuring maximum inspiratory pressure (MIP).29 Generally, there were few applicability concerns as the included studies matched most of the quality questions. The only high applicability concern was regarding the reference standard due to the incomplete information reported on respiratory mechanics in one study.29 Also, unclear applicability concern of the index test was found in one study for the lack of sufficient reporting of sEMG of extra-diaphragmatic muscles.28
This review is limited by couple of factors that affect the quality of evidence of using sEMG of extra-diaphragmatic muscles as an assessment tool of respiratory responses during MV. The included studies had small sample sizes and, thus, the evidence of usefulness of this tool across a broad population of patients on MV is limited. Additionally, there is a lack of a systematic and well-designed approach for assessing sEMG diagnostic performance, which mainly includes: random sampling of patients, blinding to index test and reference standards, and the use of gold standard reference tests for assessing MV outcomes (i.e., rapid shallow breathing index (RSBI) and MIP).
Future directions: The findings of this review may stimulate further research to test the accuracy of sEMG as a clinical diagnostic technique, which might help in the decision making of MV liberation. Further studies addressing its diagnostic accuracy should aim to examine its performance in predicting MV outcomes such as MV liberation success/failure. Additionally, studies should investigate its cost and complexity in comparison with other standard methods of MV monitoring used in the critical care settings.
ConclusionThe use of sEMG of extra-diaphragmatic muscles appears to be a valid monitoring tool with low applicability concerns for assessing respiratory mechanical loading/unloading and respiratory sensation during MV. However, high risk of bias was associated with the identified studies in introducing this technique as an assessment tool. This quality flaw was mainly attributed to the fact that these studies were not primarily designed to evaluate sEMG diagnostic accuracy of MV monitoring.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.
Authors contribution- -
Both Mr. AbuNurah & Dr. Lowman contributed to the review search, data collection, study design, analysis of data, and manuscript preparation. Dr. Russell contributed to the manuscript preparation, review and editing.
- -
This study was performed at the Department of Physical Therapy, School of Health Professions, University of Alabama at Birmingham, Birmingham, Alabama.
- -
No potential conflict of interest relevant to this article was reported.
- -
King Saud bin Abdulaziz University for Health Sciences, KSA, supported Mr. AbuNurah's work on the present study.