Non-small cell lung cancer (NSCLC) is the most common cause of cancer death worldwide, with low survival (6–12 months and overall 5-year survival 5–10%), mostly because it is usually diagnosed in advanced stages.1
In NSCLC, the most common epidermal growth factor receptor (EGFR) mutations are exon 19 and exon 21, highly associated with sensitivity to tyrosine kinase inhibitors (TKI).2 EGFR mutations are more common in non-smokers, women, adenocarcinomas and Asians patients.1 Erlotinib is an orally active and potent TKI3 indicated for first-line and maintenance treatment of patients with locally advanced or metastatic NSCLC with EGFR activating mutations.
This study (NCT01260181) was a phase II, non-randomized, open-label study to evaluate the anti-tumoral activity of erlotinib in patients with locally advanced or metastatic NSCLC with EGFR activating mutations. It was designed to evaluate the efficacy of erlotinib first-line treatment–as a single daily oral dose of 150 mg, until disease progression or unacceptable toxicity, death or withdrawal of consent–evaluated by complete or partial objective response rate (ORR) in Portugal.
This event-driven study was set up in 9 hospitals from January 2011 (start of recruitment period) to September 2017 (last safety follow-up visit).
There were 216 patients screened, 30 were enrolled, positive for exon 19 (40.0%) and/or exon 21 mutations (60.0%). Twenty-nine (96.7%) completed the treatment. At the end one patient was alive.
Patients’ mean age was 66.3 ± 9.21 years, 80% were female. Most were non-smokers (76.7%) with 20 pack-year smoking history. Median disease duration was 1 month (0–32 months) and histology for 96.7% of patients was adenocarcinoma. TNM classification was mostly stage IV (1 patient stage IIIB). Fifty percent of patients had bone metastatic disease and 80% another metastasis location. Two hundred and sixteen comorbidities have been reported and all patients had at least one. At screening, all patients had ECOG performance status 0 (20.0%), 1 (66.7%) or 2 (13.3%).
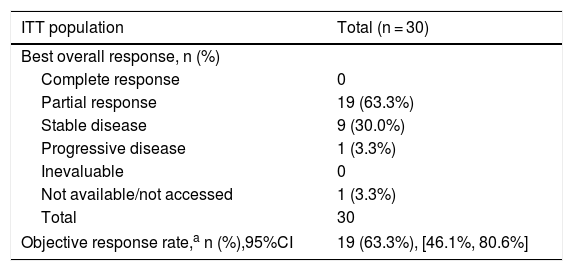
Efficacy results showed an ORR of 63.3% (95% CI: 46.1%–80.6%), in intention-to-treat, and 75.0% (95% CI: 50.1%–99.5%), in per-protocol population. The stable disease was the best overall response for the anti-tumoral activity in 30.0% of patients and progressive disease was the best overall response in 3.3%. The best overall response is summarized in Table 1.
Best overall response (RECIST v1.1 criteria) for the study populations.
| ITT population | Total (n = 30) |
|---|---|
| Best overall response, n (%) | |
| Complete response | 0 |
| Partial response | 19 (63.3%) |
| Stable disease | 9 (30.0%) |
| Progressive disease | 1 (3.3%) |
| Inevaluable | 0 |
| Not available/not accessed | 1 (3.3%) |
| Total | 30 |
| Objective response rate,a n (%),95%CI | 19 (63.3%), [46.1%, 80.6%] |
| PP population | Total (n = 12) |
|---|---|
| Best overall response, n (%) | |
| Complete response | 0 |
| Partial response | 9 (75.0%) |
| Stable disease | 3 (25.0%) |
| Progressive disease | 0 |
| Not evaluable | 0 |
| Not available/not accessed | 0 |
| Total | 12 |
| Objective response rate,a n (%), 95%CI | 9 (75.0%), [50.1%, 99.5%] |
Note: For the 7 patients without information of overall response at the end of the study visit, best overall response was obtained according to the information from the study treatment visits available.
95%CI: 95% confidence interval; ITT: intention to treat; PP: per protocol.
Median progression-free survival (PFS) was 10 months (95% CI: 7.8–15.8), and median overall survival was 20.8 months (95% CI: 14–31.2). The median duration of response was 10.4 months (95% CI: 8–15.8). For exon 19 patients, the median PFS was 14.4 months (95% CI: 4.2–41.8) and for exon 21 9.8 months (95% CI: 3.8–13.5).
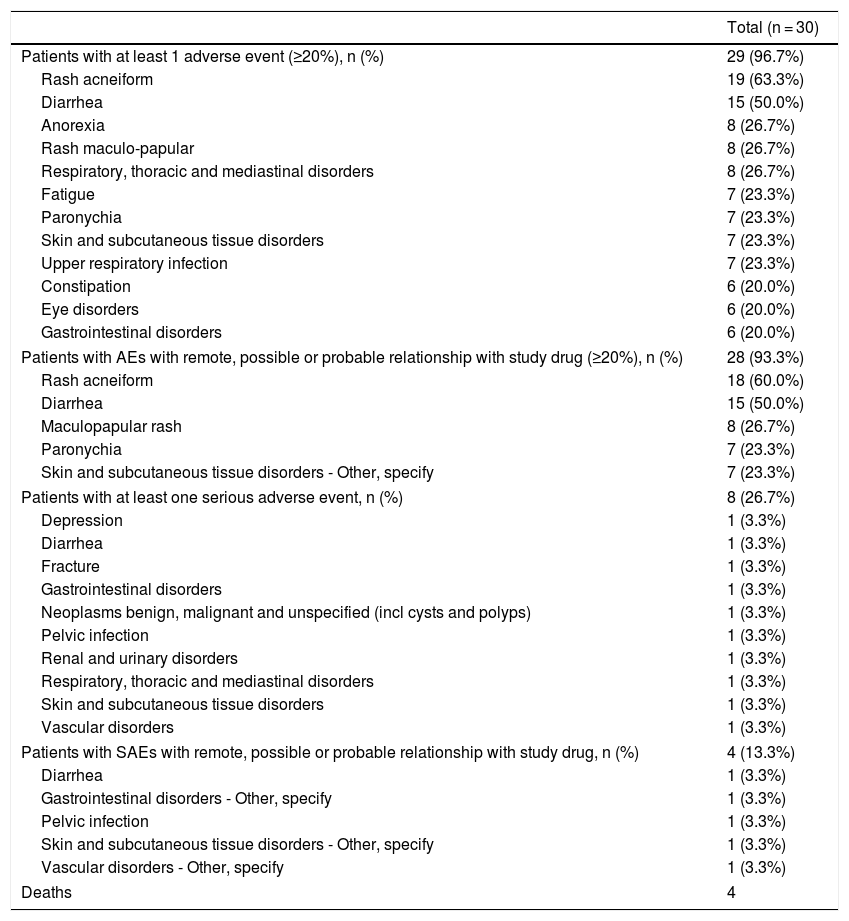
Safety profile was as expected, with 341 adverse events reported by 29 patients. The most common AEs were rash acneiform (63.3% of patients) and diarrhea (50.0%) (Table 2). From these, 49.0% were at least remotely related to treatment. More than 65% of AEs were recovered/resolved while more than one third were not recovered/not resolved, and 3 (0.9%) were fatal. There were 10 SAEs experienced by 8 patients (26.7%), 5 of them were at least remotely related to study drug (diarrhea, gastrointestinal disorders, pelvic infection, skin and subcutaneous tissue disorders and vascular disorders). One AE (interstitial lung disease) was of special interest.
Incidence of AEs and SAEs in the safety population.
| Total (n = 30) | |
|---|---|
| Patients with at least 1 adverse event (≥20%), n (%) | 29 (96.7%) |
| Rash acneiform | 19 (63.3%) |
| Diarrhea | 15 (50.0%) |
| Anorexia | 8 (26.7%) |
| Rash maculo-papular | 8 (26.7%) |
| Respiratory, thoracic and mediastinal disorders | 8 (26.7%) |
| Fatigue | 7 (23.3%) |
| Paronychia | 7 (23.3%) |
| Skin and subcutaneous tissue disorders | 7 (23.3%) |
| Upper respiratory infection | 7 (23.3%) |
| Constipation | 6 (20.0%) |
| Eye disorders | 6 (20.0%) |
| Gastrointestinal disorders | 6 (20.0%) |
| Patients with AEs with remote, possible or probable relationship with study drug (≥20%), n (%) | 28 (93.3%) |
| Rash acneiform | 18 (60.0%) |
| Diarrhea | 15 (50.0%) |
| Maculopapular rash | 8 (26.7%) |
| Paronychia | 7 (23.3%) |
| Skin and subcutaneous tissue disorders - Other, specify | 7 (23.3%) |
| Patients with at least one serious adverse event, n (%) | 8 (26.7%) |
| Depression | 1 (3.3%) |
| Diarrhea | 1 (3.3%) |
| Fracture | 1 (3.3%) |
| Gastrointestinal disorders | 1 (3.3%) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 1 (3.3%) |
| Pelvic infection | 1 (3.3%) |
| Renal and urinary disorders | 1 (3.3%) |
| Respiratory, thoracic and mediastinal disorders | 1 (3.3%) |
| Skin and subcutaneous tissue disorders | 1 (3.3%) |
| Vascular disorders | 1 (3.3%) |
| Patients with SAEs with remote, possible or probable relationship with study drug, n (%) | 4 (13.3%) |
| Diarrhea | 1 (3.3%) |
| Gastrointestinal disorders - Other, specify | 1 (3.3%) |
| Pelvic infection | 1 (3.3%) |
| Skin and subcutaneous tissue disorders - Other, specify | 1 (3.3%) |
| Vascular disorders - Other, specify | 1 (3.3%) |
| Deaths | 4 |
There were 4 deaths in the study, one due to pneumonia (not related to the study drug), one due to intestinal perforation (possibly related to the study drug), one unexplained (not related to the study drug) and the other due to progressive disease. At the end of study 51.9% of the patients had a subsequent therapy for NSCLC.
In our study, erlotinib has shown results similar to other published clinical trials in first-line treatment.4,5 In EURTAC study of first-line erlotinib versus standard IV chemotherapy, PFS was significantly longer in erlotinib-treated patients (10.4 months) than chemotherapy patients (5.1 months). Another phase-II study4 in Caucasians reinforced erlotinib as a first-line treatment of choice, with median PFS of 11 months, clinical benefit rate of 81% and median OS of 23 months, in line with our results.
In BELIEF study,6 of erlotinib 150 mg/day + intravenous bevacizumab 15 mg/kg/21 days, PFS was 13.2 months. In another double-blind, phase 3 trial in untreated patients with advanced NSCLC, EGFR mutation-positive for exon 19/21, assigned to osimertinib 80 mg/day or standard EGFR-TKI (gefitinib 250 mg od/erlotinib 150 mg/day), median PFS was 10.2 months with standard EGFR-TKIs and 18.9 months with osimertinib, with similar safety profile. The first-line bevacizumab + erlotinib versus erlotinib alone (BEVERLY study) is still waiting for results.
Safety profile was according to previous published clinical trials,3,4,6,7 with rash and diarrhea being the most commonly reported, at mild/moderate intensity.
In conclusion, erlotinib has shown to be effective and well tolerated in Portuguese NSCLC EGFR mutated patients, with locally advanced or metastatic stages. Our findings support erlotinib to be considered as first-line therapy option for locally advanced or metastatic NSCLC with EGFR-activating mutation in Portugal.
Authors contributionsFernando Barata contributed to the conception and design of the study.
Fernando Barata, Henrique Queiroga, Encarnação Teixeira, Teresa Almodovar, Marta Soares, Barbara Parente, Juan Carlos Mellidez, Paula Alves contributed to acquisition of data.
Fernando Barata, Ana Antunes contributed to analysis and interpretation of data.
Fernando Barata, Ana Antunes contributed to drafting the article.
Fernando Barata, Henrique Queiroga, Encarnação Teixeira, Teresa Almodovar, Marta Soares, Barbara Parente, Juan Carlos Mellidez, Paula Alves, Ana Antunes contributed to revising the article critically for important intellectual content.
All the authors contributed to the final approval of the version to be submitted.
FundingThis work was supported by Roche.
Conflict of interestsThe authors, F. Barata, H. Queiroga, E. Teixeira, T. Almodovar, M. Soares, B. Parente, J.C. Mellidez, P. Alves, declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
A. Antunes declares herself to be employee of Roche Farmacêutica Química which can be perceived an competing interest.
Medical writing and editorial assistance were provided by Catarina Alves, Medical Writer at Eurotrials, Scientific Consultants (now part of CTI Clinical Trial & Consulting Services), funded by Roche. Statistical support was provided by Vera Vicente, Senior Statistician at Eurotrials, Scientific Consultants (now part of CTI Clinical Trial & Consulting Services) and funded by Roche.