In December 2019, pneumonia associated with a novel coronavirus (COVID-19) was reported in Wuhan, China. Acute respiratory distress syndrome (ARDS) is the most frequently observed complication in COVID-19 patients with high mortality rates.
Objective of studyTo observe the clinical effect of plasmapheresis on excessive inflammatory reaction and immune features in patients with severe COVID-19 at risk of ARDS.
Materials and methodsIn this single-center study, we included 15 confirmed cases of COVID-19 at Masih Daneshvari Hospital, in March 2020 in Tehran, Iran. COVID-19 cases were confirmed by RT-PCR and CT imaging according to WHO guidelines. Plasmapheresis was performed to alleviate cytokine-induced ARDS. The improvement in oxygen delivery (PaO2/FiO2), total number of T cells, liver enzymes, acute reaction proteins, TNF-α and IL-6 levels were evaluated.
ResultsInflammatory cytokine levels (TNF-α, IL-6), and acute phase reaction proteins including ferritin and CRP were high before plasmapheresis. After plasmapheresis, the levels of PaO2/FiO2, acute phase reactants, inflammatory mediators, liver enzymes and bilirubin were significantly reduced within a week (p < 0.05). In contrast, although the number of T helper cells decreased immediately after plasmapheresis, they rose to above baseline levels after 1 week. Nine out of fifteen patients on non-invasive positive-pressure ventilation (NIPPV) survived whilst the six patients undergoing invasive mechanical ventilation (IMV) died.
ConclusionOur data suggests that plasmapheresis improves systemic cytokine and immune responses in patients with severe COVID-19 who do not undergo IMV. Further controlled studies are required to explore the efficacy of plasmapheresis treatment in patients with COVID-19.
In December 2019 coronavirus disease (COVID-19) was first diagnosed in the city of Wuhan in China; this pandemic disease has since spread rapidly around the world.1 The lung as the main target organ of COVID-19 can cause wide spectrum of pulmonary involvement, ranging from mild, upper respiratory tract infections to life threatening lower respiratory tract infections, including the development of acute respiratory distress syndrome (ARDS).2 The pathology of COVID-19-related ARDS and its severity depends upon the patient's immune status and target organ involvement.3 Flow cytometry analysis of blood in critically ill patients with COVID-19 revealed that the CD3+, CD4+ and CD8+ cell counts were greatly reduced.4 This lymphopenia is likely to result from high plasma inflammatory mediators negatively regulating T cell survival/proliferation leading to T cell exhaustion in these patients.5 This dysregulation of T cells may lead to enhanced disease severity and a poor prognosis with a high mortality rate.6
Effective treatment of the systemic response in severe and critically ill COVID-19 patients is key to reducing the associated mortality. Various therapeutic techniques have been used to control the inflammatory storm in these patients with COVID-19-related ARDS.7 Plasmapheresis and other blood purification techniques are recommended for the treatment of critically ill patients with a high degree of systemic inflammation. Thus, in the current study we assessed the effectiveness of plasmapheresis on systemic cytokine and immune cell levels in COVID-19 patients irrespective of whether they were undergoing invasive mechanical ventilation (IMV) or non-invasive positive-pressure ventilation (NIPPV). All patients were admitted to the Masih Daneshvari Hospital, Tehran, Iran between March and April 2020. The duration of treatment and survival were also analyzed.
Patients and methodsStudy designThe study was conducted at the Masih Daneshvari Hospital which was the referral center for COVID-19 patients at the Shahid Beheshti University of Medical Sciences (Tehran, Iran) between March-April 2020. The study protocol was approved by the ethics committee of University and was registered on the Iranian Registry of Clinical Trials (www.irct.ir, IRCT20150107020592N23). Written informed consent was obtained from all study participant, fifteen critically ill adult patients with confirmed COVID-19 as determined by clinical criteria and positive RT-PCR assays. Critically ill patients were defined as those with clinical deterioration requiring admission to the intensive care unit (ICU) and who required either invasive or non-invasive ventilation, the initial blood sample was measured immediately upon the patient's admission to the ICU and prior to receiving any treatment.
Inclusion and exclusion criteria in patient selectionAll patients were older than 18 years of age and met the criteria of the Berlin definition for the diagnosis of ARDS.8 All patients with COVID-19-induced ARDS who fulfilled these criteria were included in this study. Exclusion criteria included a record of ongoing albumin allergy, neoplastic diseases, inflammatory disease, active bleeding, chronic renal and hepatic impairment, recent myocardial infraction or coronary artery bypass graft, HIV infection or severe chronic respiratory disease.
Therapeutic approachConventional treatments such as NIPPV or IMV were initially applied. Patients received either intravenous or enteral administration of high protein, low carbohydrate nutritional supplements. Furthermore, water-electrolyte imbalances, intravascular fluid replacement, body temperature control, diuresis and other symptoms were treated as required. Patients received antiviral drugs such as Favipiravir orally (1600 mg) twice on the first day and 600 mg from the second day onwards, intravenous injection (IV) of Remdesivir 200 mg as a single dose on day 1 followed by 100 mg once daily for a total duration of 5 days. Moreover, all patients who developed respiratory tract infections were treated with intravenous infusion of Vancomycin and Meropenem at standard doses.
The therapeutic effect on severe COVID-19 patients was assessed by measuring blood parameters including the ratio of partial pressure arterial oxygen and fraction of inspired oxygen (PaO2/FiO2); plasma inflammatory mediator and acute phase protein levels ;Interleukine-6 (IL-6), Tumor necrosis-alpha (TNF-α), ferritin, C reactive protein (CRP), polycalcitonine (PCT); CD3+, CD4+ and CD8 + T cell numbers and liver function tests aspartate transaminase (AST), alanine aminotransferase (ALT) and bilirubin) as well mortality. The survival time was calculated from the time of patient ICU admission to the time the patient was discharged from the hospital.
Plasma purification techniqueRemoval of inflammatory mediators from the plasma using blood purification techniques such as plasmapheresis plays a key role in the management of various diseases and may attenuate the response particularly in the early phase of ARDS.8–10 Plasmapheresis was performed whether the patient remained under IMV or NIPPV to improve O2 saturation and reduce pro-inflammatory mediator levels. Plasmapheresis was performed via femoral venous catheters at a blood flow rate of 50−120 ml/min based on the patient’s blood pressure using a JMS fully automated Sds-20 Hemodialysis Machine.11 It was implemented over six hours per day, three times weekly for all patients whose clinical condition did not improve. Blood samples were collected before and after each plasmapheresis to enable measurement of blood parameters.
During each session a volume of 40 mL/kg bodyweight of patient's plasma was exchanged with an equal volume of 5% human albumin solution and 0.9% saline. In four patients, based on their ABO blood group matching, received fresh plasma from donors with positive detection of anti-SARS-CoV-2 IgG and IgM in their whole blood in addition to albumin and saline. After the first plasmapheresis session the effectiveness was evaluated and confirmed by the presence, or progression, of hemodynamic instability and the development of organ dysfunction. Patients were followed until discharged from ICU or death.
Data collectionAll patients were assessed for sequential (sepsis-related) organ failure assessment (SOFA) and acute physiology and chronic health evaluation II (APACHE II) scores on the day of ICU admission. Whole blood samples were collected from patients before and after plasmapheresis in EDTA tubes. Plasma was isolated and then stored at -70 °C until analysis. Cytokine (TNF-α and IL-6) detection was performed by ELISA (Immolate, DPC Biermann Bad Nauheim, Germany), lymphocyte subset analysis was determined by flow cytometry analysis (FACS, Beckman Coulter, IN, USA) by using FITC-, PE- and APC-labeled antibodies for CD3, CD4 and CD8 T cells respectively (all from BD Pharmangin, CA, USA). The other biochemical parameters were determined using an automatic biochemical analyzer.
Statistical analysisThe data were analyzed using the statistical package IBM SPSS version 24.0 and descriptive statistics (Statistical Package for the Social Sciences, Chicago, IL). The categorical variables are expressed as proportions and frequencies. The Kolmogorov–Smirnov test (KS test) was used to test normality of continuous variables. Normally distributed continuous variables are summarized as means and standard deviations. The paired t-test and Mann-Whitney U test were used to compare the mean/median between two groups. P values <0.05 were considered significant.
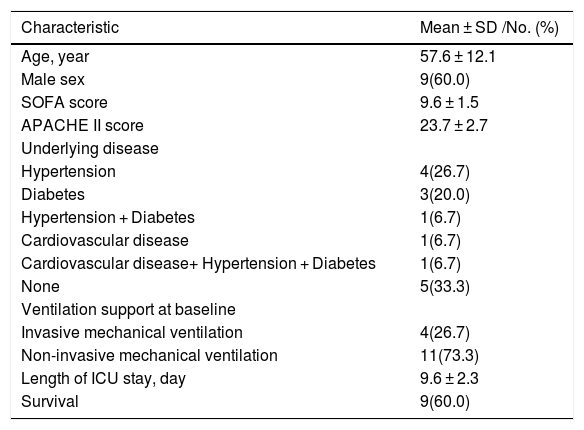
ResultsPatient characteristicsTable 1 shows the demographic information of participating COVID-19 infected patients. The mean age of the patients was 57.6 ± 12.1 years. Nine (60%) patients were male and six (40%) were female. At baseline the mean of APACHE II and SOFA scores were 23.7 ± 2.7 and 9.6 ± 1.5, respectively. Ten patients had comorbidities including hypertension (HTN, 26.7%), diabetes mellitus (DM, 20.0%), HTN + DM (6.7%), cardiovascular (6.7%) and cardiovascular + DM + HTN (6.7%). All patients had respiratory involvement at admission, four patients with PaO2 /FiO2 lower than 100 urgently required IMV whilst NIPPV was used for the other subjects. During the study, two patients on NIPPV developed severe hypoxemia requiring intubation and mechanical ventilation.
Demographics and baseline characteristics of patients infected with COVID-19 (n = 15).
| Characteristic | Mean ± SD /No. (%) |
|---|---|
| Age, year | 57.6 ± 12.1 |
| Male sex | 9(60.0) |
| SOFA score | 9.6 ± 1.5 |
| APACHE II score | 23.7 ± 2.7 |
| Underlying disease | |
| Hypertension | 4(26.7) |
| Diabetes | 3(20.0) |
| Hypertension + Diabetes | 1(6.7) |
| Cardiovascular disease | 1(6.7) |
| Cardiovascular disease+ Hypertension + Diabetes | 1(6.7) |
| None | 5(33.3) |
| Ventilation support at baseline | |
| Invasive mechanical ventilation | 4(26.7) |
| Non-invasive mechanical ventilation | 11(73.3) |
| Length of ICU stay, day | 9.6 ± 2.3 |
| Survival | 9(60.0) |
SOFA = Sequential Organ Failure Assessment; APACHE = Acute Physiology and Chronic Health Evaluation.
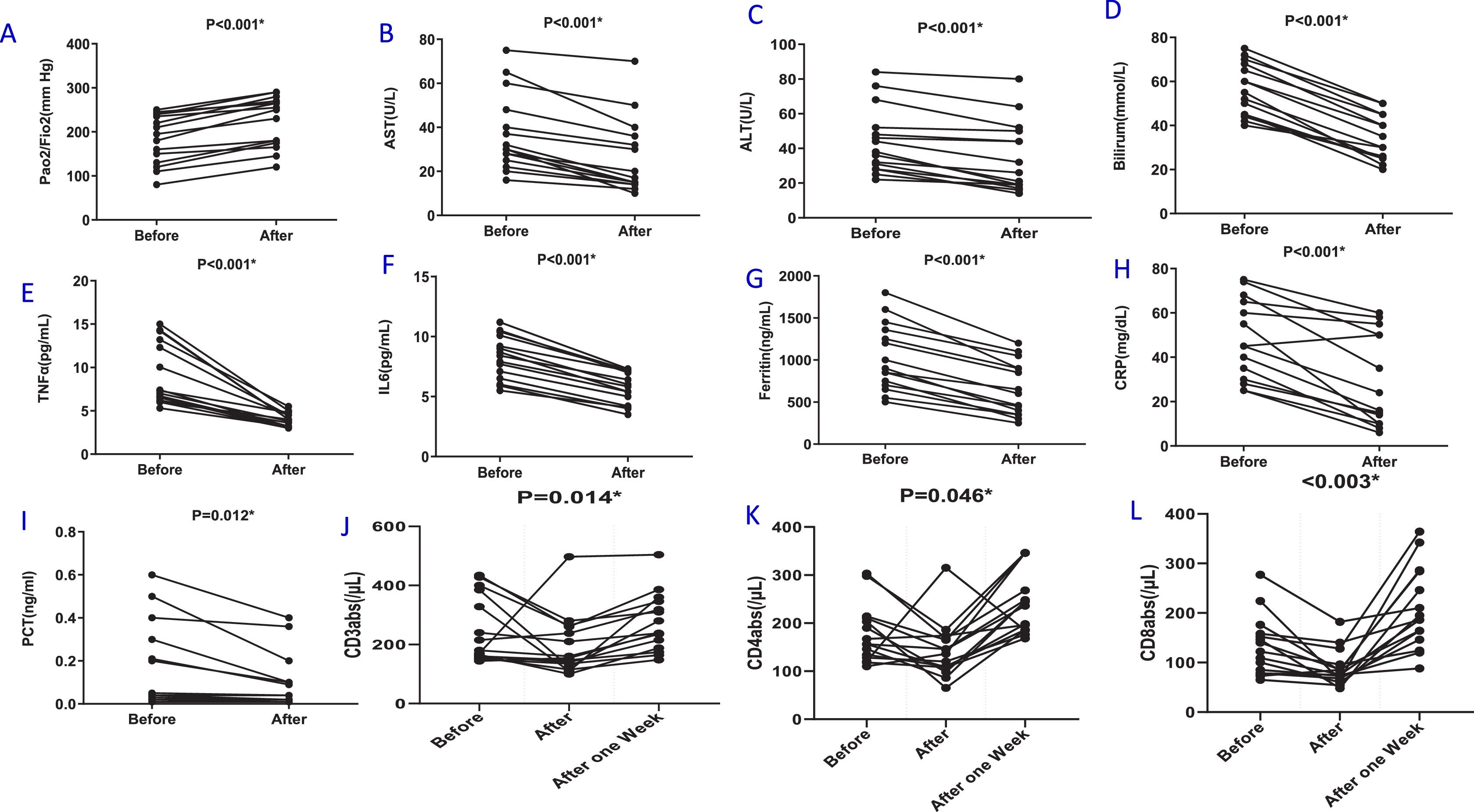
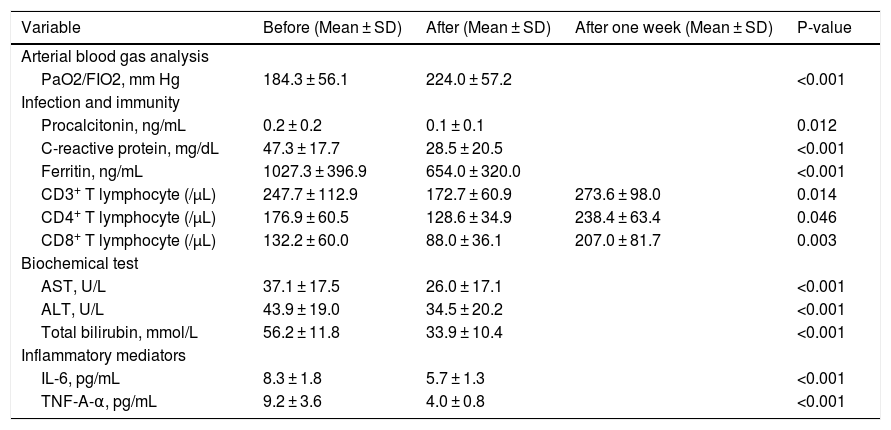
Table 2 and Fig. 1 show the oxygen status (PaO2/FiO2), blood mediator and T cell numbers before and after plasmapheresis. Plasmapheresis was associated with significant and rapid improvements in oxygenation status (p < 0.001, Fig. 1A), hepatic function (p < 0.001, Fig. 1B-D), reduced inflammatory mediators (p < 0.001, Fig. 1E-F) and acute phase reactant levels (p < 0.05, Fig. 1G-I). However, T cell subset numbers were significantly decreased (p < 0.05, Fig. 1J-L).
Laboratory findings of patients infected with COVID-19 on admission to ICU (n = 15).
| Variable | Before (Mean ± SD) | After (Mean ± SD) | After one week (Mean ± SD) | P-value |
|---|---|---|---|---|
| Arterial blood gas analysis | ||||
| PaO2/FIO2, mm Hg | 184.3 ± 56.1 | 224.0 ± 57.2 | <0.001 | |
| Infection and immunity | ||||
| Procalcitonin, ng/mL | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.012 | |
| C-reactive protein, mg/dL | 47.3 ± 17.7 | 28.5 ± 20.5 | <0.001 | |
| Ferritin, ng/mL | 1027.3 ± 396.9 | 654.0 ± 320.0 | <0.001 | |
| CD3+ T lymphocyte (/µL) | 247.7 ± 112.9 | 172.7 ± 60.9 | 273.6 ± 98.0 | 0.014 |
| CD4+ T lymphocyte (/µL) | 176.9 ± 60.5 | 128.6 ± 34.9 | 238.4 ± 63.4 | 0.046 |
| CD8+ T lymphocyte (/µL) | 132.2 ± 60.0 | 88.0 ± 36.1 | 207.0 ± 81.7 | 0.003 |
| Biochemical test | ||||
| AST, U/L | 37.1 ± 17.5 | 26.0 ± 17.1 | <0.001 | |
| ALT, U/L | 43.9 ± 19.0 | 34.5 ± 20.2 | <0.001 | |
| Total bilirubin, mmol/L | 56.2 ± 11.8 | 33.9 ± 10.4 | <0.001 | |
| Inflammatory mediators | ||||
| IL-6, pg/mL | 8.3 ± 1.8 | 5.7 ± 1.3 | <0.001 | |
| TNF-A-α, pg/mL | 9.2 ± 3.6 | 4.0 ± 0.8 | <0.001 |
PaO2/FIO2= arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen; AST = aspartate transaminase; ALT = alanine aminotransferase; IL-6= interleukin -6; TNF-A- α= tumor necrosis factor-α.
Effects of Plasmapheresis on cytokine and immune cell levels in COVID-19 patients with acute respiratory distress syndrome (ARDS). Oxygenation status [ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2)] was evaluated in arterial blood (A). Serum was isolated from whole blood without anticoagulant and liver function was evaluated by measuring AST (B), ALT (C) and Bilirubin (D). The serum levels of interleukin (IL)-6 (E) and TNF-α (F) were evaluated by ELISA. Acute phase proteins including Feritin (G), CRP (H) and PCT (I) were evaluated by biochemical methods. The percentage of CD3 (J), CD4 (K) and CD8 (L) T cell subsets was also recorded. Data are presented as values for each individual. P values for each comparison are indicated on the individual panels.
One week after plasmapheresis, the levels of all lymphocyte subsets increased to above the levels seen at baseline in patients who improved (p < 0.05, Table 2). The timing of the improvement in lymphocyte count was consistent with the time point of the improvement in clinical course. Overall, plasma levels of inflammatory mediators and acute phase reactants were negatively correlated with blood T cell counts in severe patients with COVID-19 pneumonia.
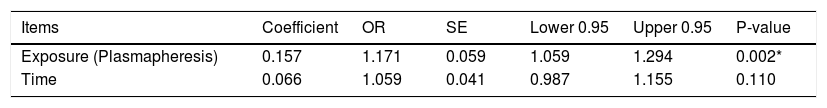
Case-crossover designA conditional logistic regression model based on adjusting the time trend was used to investigate the effect of using plasmapheresis on patient survival to eliminate confounding patient characteristics.12 Plasmapheresis was used every 48 h according to the patient's condition and the level of IL-6. The results of our case-crossover design are summarized in Table 3. Plasmapheresis has a significant effect on patient survival (P = 0.002) with an Odds Ratio (1.171) indicating that plasmapheresis increased survival by 17%. The likelihood ratio test was 59.54 with 2 degrees of freedom.
Prognosis analysisThe survival rate of patients is shown in Table 1. The primary outcome was improved clinical outcome in severe COVID-19 patients after plasmapheresis. Nine (60%) out of twelve critically ill patients with SARS-CoV-2 infection who underwent NIPPV survived. Unfortunately, the other 6 more severe COVID-19 patients on IMV died. The median length of ICU stay in patients who survived was 9.6 ± 2.3 days.
DiscussionThe current study was designed to evaluate the effect of plasmapheresis on systemic cytokine and immune cell levels in severe COVID-19 patients with ARDS. Therapeutic plasmapheresis in critically ill patients with COVID-19 reduced excess pro-inflammatory cytokine, liver function and acute phase protein levels which could help to support vital organ function. In addition, plasmapheresis improved oxygenation status and lymphocyte subset counts. Moreover, all subjects on NIPPV who underwent plasmapheresis survived. These data emphasize the need for controlled studies of plasmapheresis in patients with severe COVID-19 with ARDS.
COVID-19 pneumonia induces a progressive hypoxemia and inflammatory response which can develop into acute lung injury and multi-organ failure as a serious consequence.13 Patients with severe COVID-19 ARDS have a higher mortality rate as compared with the mortality rate commonly seen in severe ARDS resulting from other diseases.14 To date, the management of ARDS in COVID-19 remains supportive and most therapeutic interventions use re-purposed drugs and no COVID-19-specific treatments are available.15 As such severe COVID-19 patients have been treated with antimicrobial agents, antiviral, antimalarial and corticosteroid therapies with variable degrees of effectiveness.16,17
The efficacy of blood replacement therapy in removing inflammatory mediators, immune complexes and in the management of the cytokine storm has shown in multiple disorders18,19 including in critically ill COVID-19 patients.9,20,21 In addition, plasmapheresis depletes elevated levels of serum TNF-α and IL‐6 following transplantation.22 IL-1 is important in the early stage of ARDS and is associated with subsequent chemokine production and edema and targeted reduction IL-1β has been used therapeutically.23 However, high plasma levels of IL-6 and high IL-8 levels in bronchoalveolar lavage fluid are associated with a higher mortality in ARDS patients.24 Severe COVID-19 patients with ARDS have high levels of TNF-α and of IL6 particularly those requiring ICU admission suggesting that the cytokine storm might be important in severe disease.25 In addition to these pro-inflammatory cytokines, a role for anti-inflammatory cytokines such as IL-4 and IL-10 has also been postulated in COVID-19.26
Previous studies of plasmapheresis in COVID-19 patients have not been associated with detailed analysis of the molecular and biochemical mechanisms underlying the successful intervention. We show here that plasmapheresis results in a rapid reduction in TNF-α and IL-6 blood levels and that this is associated with improvement in clinical outcomes in severe COVID-19 patients on NIPPV.
Enhanced systemic levels of key liver enzymes and of slightly elevated bilirubin levels is seen in severe COVID-19 subjects rather than mild patients and is linked to levels of liver damage increasing from 14.8% to 53%.27,28 Furthermore, in fatal cases of COVID-19 the incidence of liver injury might reach as high as 58.06%29 to 78%.30 Studies in acute liver failure shows that plasma replacement reduces the amount of blood accumulated toxins and improves liver function tests.31 Plasma replacement also effectively removed systemic TNF-α and IL-6 in patients with severe acute hepatic failure.32 Our data on severe COVID-19 patients clearly demonstrated that plasmapheresis also improves liver function in these patients as evidenced by a reduction in the elevated baseline serum hepatic enzyme levels.
CD3 is an important marker of mature T lymphocytes and helps to activate the CD4+ T cell and CD8+ T cells which play an important role in antiviral immunity.33 Excessive activation of T cells during COVID-19 infection results in a dramatic T cell exhaustion evidenced by the reduced number of total blood T cells, which is, in turn, correlated with disease progression5,34 and severity of COVID-19 patients.6,35
Previous studies have reported that plasmapheresis could be a useful adjunct to mechanical ventilation in acute respiratory failure.36,37 In addition, clinical studies show that blood purification techniques, play a key role in effectively reducing the mortality of patients with severe COVID-19.7 However, the evidence to date is insufficient to recommend the routine use of plasmapheresis to correct the hypoxemia, which can lead to multiorgan dysfunction due to advanced modes of treatment.38 However, plasmapheresis may be used based on disease severity and the availability of resources. Importantly, we demonstrate that plasmapheresis improves some clinical variables in our patients such as the PaO2/FiO2 ratio although we cannot confirm a significant survival benefit due to a lack of control subjects.
Our study has several limitations. First, only 15 patients with confirmed COVID-19 were studied. Second, it was an uncontrolled single-center study; it would be useful to conduct a controlled multicenter study either in Iran or ideally across several countries to evaluate the efficacy of plasmapheresis in COVID-19 infection. In our study, plasmapheresis improves oxygenation, lymphocyte counts and demonstrated benefits in managing the cytokine storm in severe COVID-19 patients with ARDS.
Multiple testing could be an issue in the current study and even more so in transcriptomic and other unbiased omic analysis. It is possible that the results shown may reflect false positives due to multiple testing and that multiple corrections of the raw p values may provide a better reflection of the true significance of the differences reported here. However, using an FDR value may hide possible important factors and we feel that reporting the raw p values provides an opportunity to draw conclusions about the data and the need for subsequent validation. Since this disease is new and has many unknown dimensions, in this study and other ongoing studies, all possible factors and indicators that may change due to this disease were examined.
We were unable to demonstrate an effect on mortality as only our less severe COVID-19 patients using NIPPV survived and survival may reflect the severity of disease and/or the effect of NIPPV. All subjects who required IMV and had plasmapheresis died which again may reflect the severity of the disease. It is important to perform controlled studies to delineate the effect of the intervention per se.
To conclude, our study demonstrated that the therapeutic plasmapheresis as a blood purification technique offers safety and efficacy in removal of inflammatory cytokines, acute phase proteins and improves tissue oxygenation.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all patients, healthcare workers, and clinical pharmacists involved in the study.



![Effects of Plasmapheresis on cytokine and immune cell levels in COVID-19 patients with acute respiratory distress syndrome (ARDS). Oxygenation status [ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2)] was evaluated in arterial blood (A). Serum was isolated from whole blood without anticoagulant and liver function was evaluated by measuring AST (B), ALT (C) and Bilirubin (D). The serum levels of interleukin (IL)-6 (E) and TNF-α (F) were evaluated by ELISA. Acute phase proteins including Feritin (G), CRP (H) and PCT (I) were evaluated by biochemical methods. The percentage of CD3 (J), CD4 (K) and CD8 (L) T cell subsets was also recorded. Data are presented as values for each individual. P values for each comparison are indicated on the individual panels. Effects of Plasmapheresis on cytokine and immune cell levels in COVID-19 patients with acute respiratory distress syndrome (ARDS). Oxygenation status [ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2)] was evaluated in arterial blood (A). Serum was isolated from whole blood without anticoagulant and liver function was evaluated by measuring AST (B), ALT (C) and Bilirubin (D). The serum levels of interleukin (IL)-6 (E) and TNF-α (F) were evaluated by ELISA. Acute phase proteins including Feritin (G), CRP (H) and PCT (I) were evaluated by biochemical methods. The percentage of CD3 (J), CD4 (K) and CD8 (L) T cell subsets was also recorded. Data are presented as values for each individual. P values for each comparison are indicated on the individual panels.](https://static.elsevier.es/multimedia/25310437/0000002700000006/v1_202111060605/S2531043720302543/v1_202111060605/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)




