Optic neuromyelitis (NMO) is an autoimmune inflammatory disorder of the central nervous system characterized by episodes of immune-mediated demyelination and axonal damage mainly involving optic nerves and spinal cord.1 Immunoglobulin (Ig)G antibodies against the water channel protein aquaporin 4 (AQP4) play a pivotal role in the pathogenesis of the disease. Although most commonly an idiopathic autoimmune condition, it may also occur as a paraneoplastic syndrome and/or associated with other autoimmune diseases such as Sjögren’s syndrome, sarcoidosis, antiphospholipid syndrome and systemic lupus erythematosus. An unusual case of organizing pneumonia in a patient with NMO is reported herein. These clinical entities might share common pathogenic mechanisms, as suggested by the present study which could explain their co-existence in the patient.
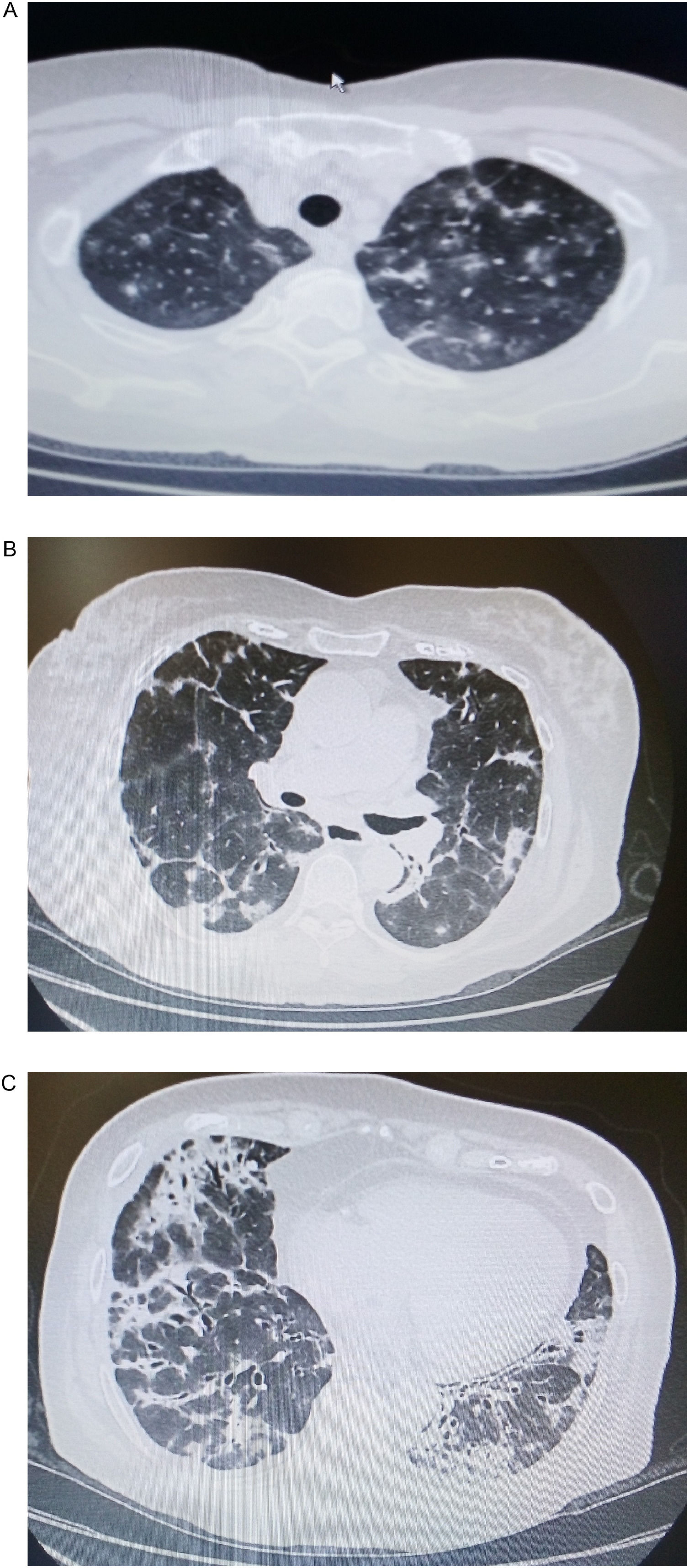
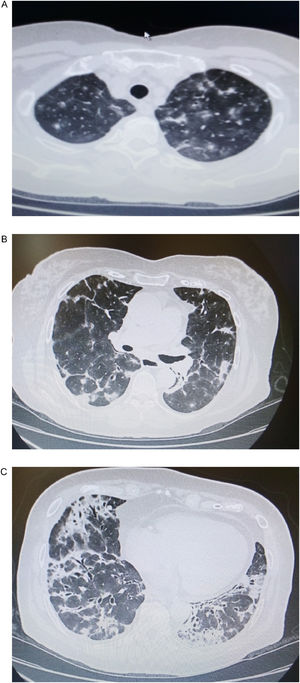
A 77 year old woman, non-smoker, was admitted to our department presenting dyspnea on exertion, productive cough and low-grade fever (37,8°C) for two months before her admission. Physical examination of the chest revealed fine crackles throughout the lung fields. Upon admission, a chest CT showed bilateral multifocal asymmetric consolidations with predominantly subpleural distribution, especially in both upper and middle lung fields. Ground glass appearance is also evident (Fig. 1a, b). In addition, bilateral consolidations with strongly peribronchovascular distribution and dilated airways within them were noticeable in the lower lung fields (Fig. 1c). Standard laboratory results demonstrated mild leukocytosis and hypoxemia (WBC count: 12,200/μL, neutrophils: 10,600/μL, pO2: 68mmHg), as well as severely elevated creatine phosphokinase levels (2048IU/L).
(a, b) Chest CT showing bilateral multiple consolidations with distribution accompanied by ground glass opacities throughout the upper and middle lung fields. (c) Bilateral consolidations with peribronchovascular distribution and dilated airways within them are visible in the lower lung fields.
Fiberoptic bronchoscopy was performed and BAL fluid analysis revealed a mixed cellularity pattern with 28% lymphocytes, 16% neutrophils, 12% eosinophils, 4% mast cells, 40% macrophages and CD4/CD8 ratio of 0.12. Microbiological and cytological examinations were negative. Conventional transbronchial lung biopsies were non-diagnostic. However, BAL findings were highly suggestive of organizing pneumonia.2
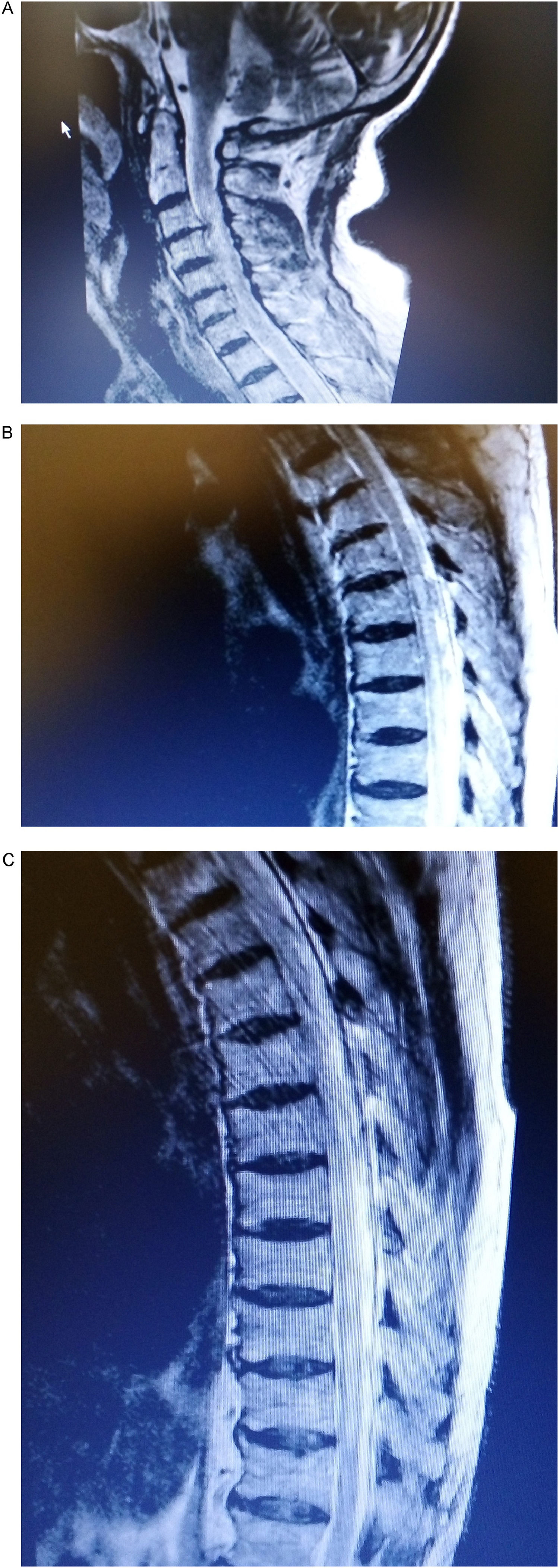
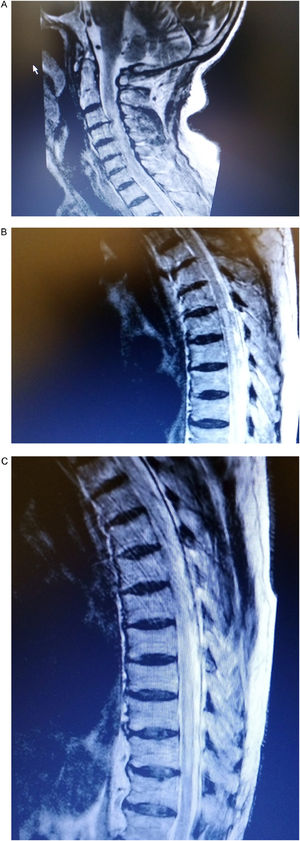
Furthermore, the patient experienced numbness, paresthesia and partially affected mobility of both lower limbs and left upper limb on the second day of hospitalization. Neurologic examination revealed a sensitive thoracic level T6−9, hyperactive bilateral patellar tendon reflexes and a 4/5 grade muscle strength. Lumbar puncture disclosed lymphocytic pleocytosis and absent oligoclonal bands. PCR test for detecting viruses in the cerebrospinal fluid was negative. Cervical and thoracic spine MRI showed high intensity signals at the C1–2, C4–7 levels and T6–9 on T2-weighted imaging, suggestive of an extensive acute transverse myelitis (Fig. 2a–c). Additionally, serum AQP4 (aquaporin) IgG levels were greatly increased and the diagnosis of NMO was established. The patient was treated with high-dose corticosteroids exhibiting gradual improvement of neurologic deficits and resolution of pulmonary infiltrates within a three-month period. He is still on tapering course of steroids with a schedule of rituximab initiation as maintenance therapy.
It is widely recognized that NMO is mainly antibody-mediated with the principal role played by the humoral immune system targeting astrocytes. More specifically, a circulating IgG autoantibody against the water channel protein aquaporin-4 (AQP4) expressed by astrocytes has been established as the major key factor in the pathogenesis of this disorder. The positivity for IgG-AQP4 antibodies is a robust criterion for NMO diagnosis in conjunction with typical clinical and MRI features.1 The created IgG-AQP4 complex downregulates the surface expression of AQP4 in the central nervous system cells causing increased blood-brain barrier permeability, activating the complement, promoting the accumulation of inflammatory cells and leading to astrocyte damage and death.3
OP is an inflammatory lung disease that is characterized by the presence of buds of granulation tissue in the lumen of the distal pulmonary airspaces as a repair process of the lung in response to preceding alveolar injury.4 The coexistence of NMO and OP in the present case might not be coincidental. The authors allege that AQP4 plays a pivotal role in this relationship. There is a growing body of evidence from studies on lung diseases that AQPs are involved especially in those lung diseases that are caused or at least accompanied by perturbed airway surface liquid volume homeostasis like asthma, COPD and acute lung injury.5 Furthermore, the recognized role of AQPs in inflammatory cell infiltration, cell proliferation and migration may account for their evolving role in lung inflammatory disorders and various lung cancers. In particular, downregulation of AQP4 in alveolar epithelium thus leading to lung inflammation (increased vascular permeability, cellular infiltration, cytokine release) has recently been demonstrated in lung models.6
There is a paucity of literature concerning pulmonary complications associated with NMO and especially interstitial lung diseases. Strictly speaking, 5 cases have hitherto been published dealing with NMO-related ILDs (sarcoidosis, cryptogenic organizing pneumonia, and unclassified interstitial pneumonia).7–10 However, the diagnosis of OP in 3 out of 5 cases was based solely on clinical grounds without confirmation by using bronchoscopic procedures (BAL/transbronchial biopsy).
In summary, OP could be associated with NMO. Although a causal association cannot be explicitly ascertained from this descriptive study, we suggest that AQP4 downregulation in astrocytes may also orchestrate the inflammatory process in organizing pneumonia due to its expression in lung tissue. However, more data are necessary to clarify the relationship, bearing in mind the small number of patients reported with this combination of diseases.
FundingNone.
Conflicts of interestThe authors have no conflicts of interest to declare.
Authors’ contributionTR was responsible for patient management and prepared the manuscript. AA and NF helped to confirm optic neuromyelitis and drafted the manuscript. SK and KP critically revised the manuscript and approved the final version. All authors have read and approved the final manuscript and agreed to be accountable for all aspects of the work.