Chronic Obstructive Pulmonary Disease (COPD) history is characterized by episodes of exacerbation of varying severity, featured by acute worsening of respiratory symptoms, commonly precipitated by respiratory tract infection. The recent ERS/ATS clinical practice guidelines strongly recommend the application of non invasive ventilation (NIV) for patients with acute respiratory failure (ARF) leading to acute or acute-on-chronic respiratory acidosis (pH 7.35) and not for those patients with acute exacerbation of COPD (AECOPD) and hypercapnia who are not acidotic. In recent years, High-Flow through Nasal Cannula (HFNC) has been introduced in the clinical practice.
We designed the present systematic review of the literature to assess all effects of HFNC use reported in exacerbated COPD patients. In this setting, HFNC is able to keep PaCO2 unmodified, while oxygenation slightly deteriorates as opposed to NIV. Furthermore, the work of breathing is reduced with HFNC by a similar extent to NIV, while it increases by 40–50% during conventional oxygen therapy (COT). HFNC is also reported to be more comfortable than COT and NIV. Despite these results, little and limited evidence for improved clinical outcomes is currently available.
Chronic Obstructive Pulmonary Disease (COPD) is a common disease characterized by persistent respiratory symptoms and airflow limitation due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.1
COPD history is characterized by episodes of exacerbation of varying severity, featured by acute worsening of respiratory symptoms, commonly precipitated by respiratory tract infection. The most severe exacerbation episodes may be associated with acute respiratory failure (ARF) deeming conventional oxygen therapy (COT) necessary, in case of sole hypoxemia, or invasive or non-invasive (NIV) ventilation, in case of carbon dioxide (CO2) retention and respiratory acidosis.2 Indeed, NIV has been shown to improve gas exchange, to reduce work of breathing and the need for intubation, to decrease hospital length of stay and mortality.2 The recent ERS/ATS clinical practice guidelines strongly recommend the application of NIV for patients with ARF leading to acute or acute-on-chronic respiratory acidosis (pH ⩽ 7.35) and not for those patients with acute exacerbation of COPD (AECOPD) and hypercapnia who are not acidotic.2
However, NIV may fail in up to 64% of exacerbated COPD patients for several reasons, and invasive mechanical ventilation is instituted.3 The two main causes of NIV failure include worsened respiratory function and mask intolerance, followed by cardiovascular instability and neurological deterioration.3
In the recent years, High-Flow through Nasal Cannula (HFNC) has been introduced in the clinical practice. HFNC delivers to the patient heated humidified air-oxygen mixture, with an inspiratory fraction of oxygen (FiO2) ranging from 21 to 100% and a flow up to 60 L/min through a large bore nasal cannula.4,5 HFNC has some potential advantages for COPD patients.5 First of all, HFNC provides heated (37 °C) and humidified (44 mg/L) air-oxygen admixture to the patient, which avoids injuries to ciliary motion, reduces the inflammatory responses associated to dry and cold gases, epithelial cell cilia damage, and airway water loss, and keeps unmodified the water content of the bronchial secretions.5 Second, HFNC determines a wash out from CO2 of the pharyngeal dead space (comprising ∼30% of the overall anatomical dead space), which is relevant for patients with an incremented ratio between dead space and tidal volume, such as in COPD patients.5 Third, HFNC generates a small amount (up to 8 cmH2O) of pharyngeal pressure during expiration, which drops to zero during inspiration.5,6 This effect resembles the pursed-lip breathing pattern, a strategy adopted by COPD patients to diminish the respiratory rate and to prolong the expiratory time and to reduce the expiratory flow limitation and dynamic hyperinflation.5 Fourth, HFNC guarantees a more stable FiO2, as compared to COT. Whenever the inspiratory peak flow of a patient exceeds the flow provided by a Venturi mask, the patient inhaled also part of atmospheric air.7 In exacerbated COPD patients, the mean inspiratory peak flow has been reported to be around 70 L/min and to exceed 60 L/min in about 70% of the patients.8 Therefore, the use of HFNC may guarantee a more stable FiO2, as compared to COT through a Venturi mask. Lastly, HFNC assures optimal comfort compared to both COT7 and NIV.9 Based on these potential advantages, HFNC might be used in exacerbated COPD patients with different indications.
We therefore designed the present systematic review of the literature to assess all effects of HFNC use reported in exacerbated COPD patients.
Materials and methodsSearch methods for identification of studiesWe carried out an electronic search of Medline, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central and Scopus from inception until June 10th, 2019. We launched the following search strategy in Medline: “("respiratory failure" [MeSH Terms] OR "acute respiratory failure" [All Fields] OR "respiratory insufficiency" [All Fields] OR "respiratory failure" [All Fields] OR "COPD" [All Fields] OR "Chronic Obstructive Pulmonary Disease" [All Fields] OR "chronic respiratory failure" [All Fields] OR "hypercapnic" [All Fields] OR “Hypercapnic Acute Respiratory Failure” [All Fields] OR "acute on chronic respiratory failure" [All Fields]) AND ("high flow nasal oxygen" [All Fields] OR "high-flow nasal oxygen" [All Fields] OR "high flow nasal cannula" [All Fields] OR “high-flow nasal cannula" [All Fields] OR "high flow oxygen" [All Fields] OR "high-flow oxygen" [All Fields] OR “High-Flow Oxygen Therapy” [All Fields])”. Medline search strategy was adapted for searches in other databases.
Controlled vocabulary terms (when available), text words, and keywords have been variably combined.
No filters were applied.
Studies selectionWe have included randomized or non-RCTs (including crossover design) comparing HFNC with a control group (both COT or NIV). Studies have been considered only if including adult (>18 years/old) COPD patients receiving HFNC and hospitalized because of an episode of COPD exacerbation. Titles and abstracts have been independently screened by two authors (FL and LP) according to the inclusion criteria and the full texts of the potentially relevant reports have been retrieved. The full-text reports have been independently examined by two authors (FL and LP).
We have excluded case reports, case series, retrospective or observational studies, reviews, editorial and commentaries. We also excluded all studies not written in English language. Included studies have been recorded using a Microsoft Excel standardized report form.
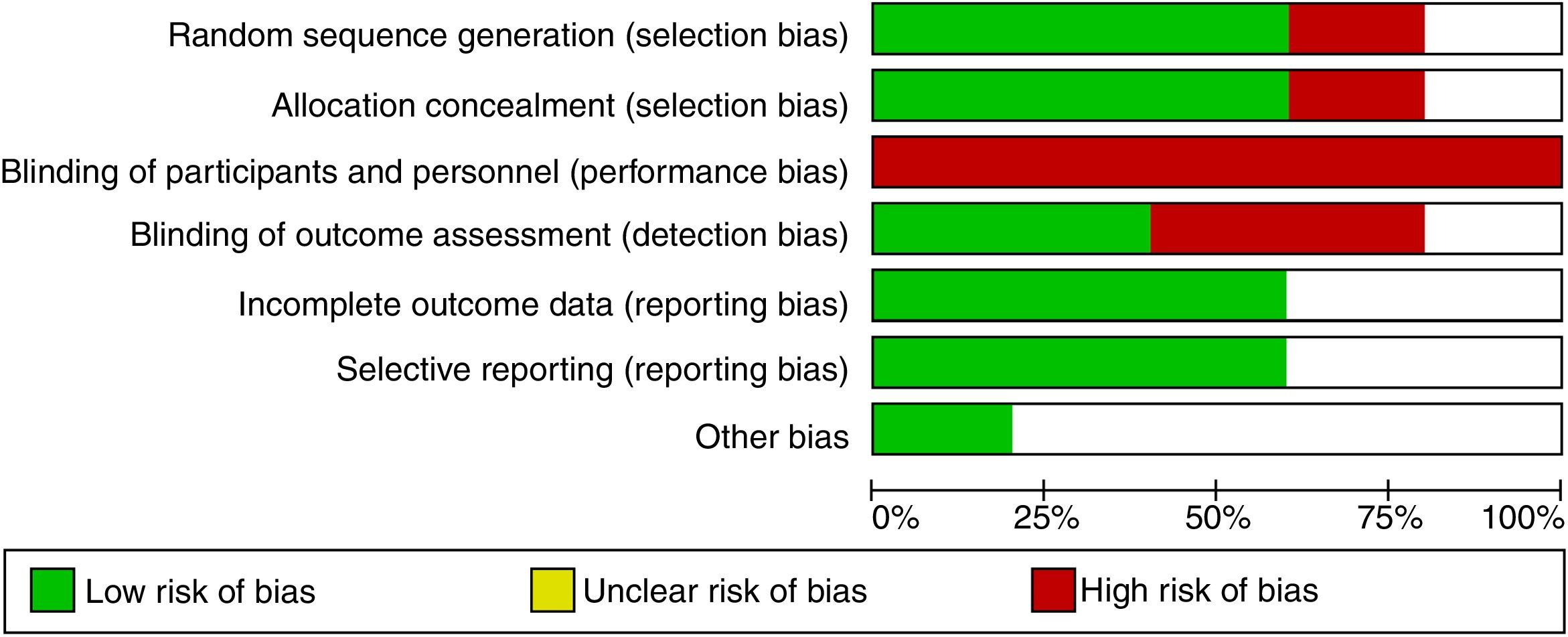
Risk of bias assessmentWe have evaluated the methodological quality of all studies for randomized sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias. Randomized and nonrandomized crossover studies have been assessed according to a modified version of the checklist proposed by Ding et al.10 Review Manager software (RevMan 5.3; Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) has been used for the assessment and graphical editing.
Statistical analysisStatistical analysis was conducted on the summary statistics reported in the selected articles. On this basis, the statistical unit of observation for all the selected variables was the single study and not the patient. Descriptive statistics of individual studies used different statistical indicators for central tendency and variability.11,12 When data were reported as median in the selected studies, we estimated the sample mean and standard deviation of the population, as previously described.13 We therefore compute the median of the means of the study for some variable of interest. Data are therefore reported as means and standard deviations (SD) or median [25th–75th interquartile], as appropriate. No meta-analyses on summary findings or on individual patient data were performed.
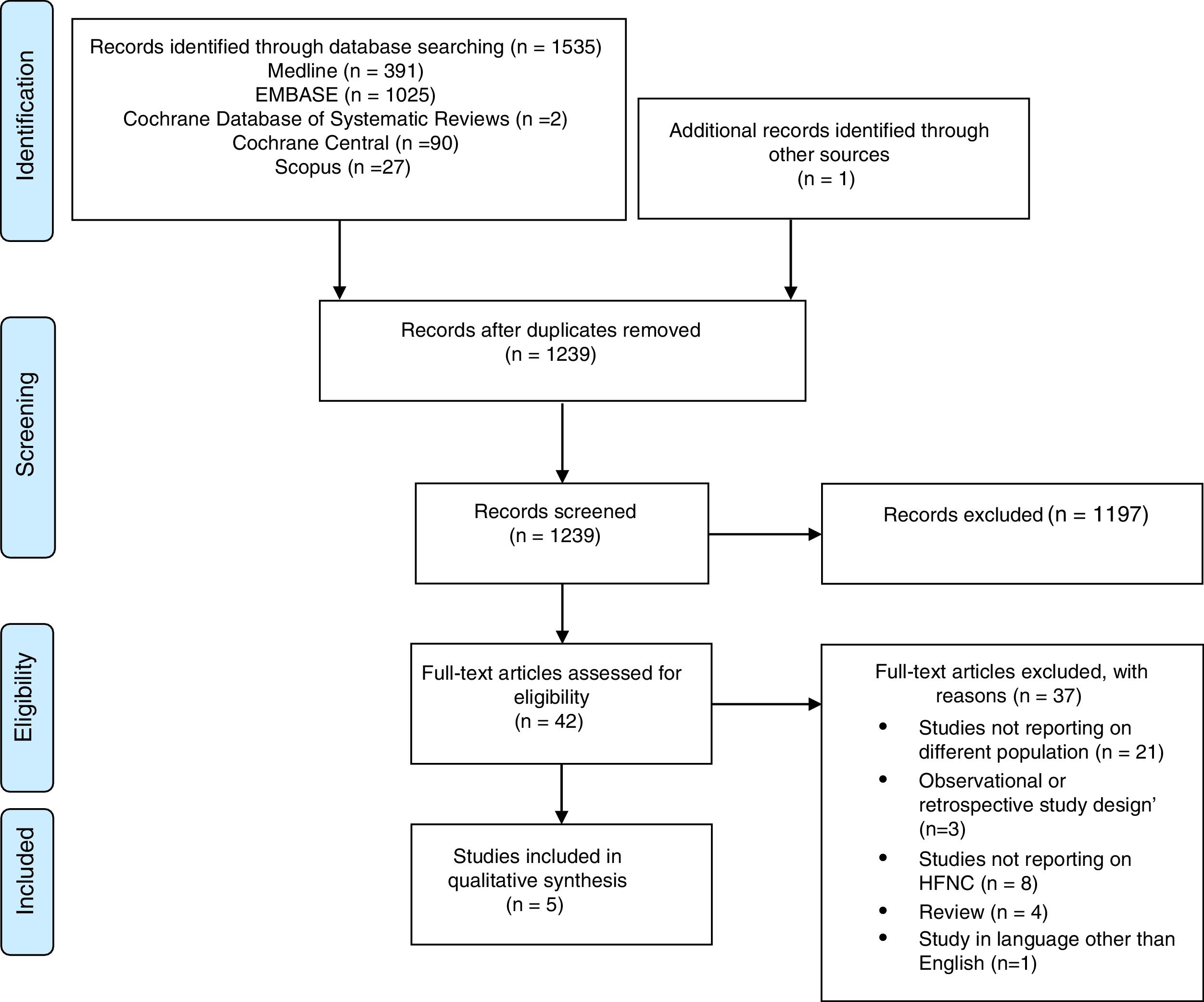
ResultsThe electronic search has totally identified 1535 potentially relevant studies (391 from Medline, 1025 from EMBASE, 2 from Cochrane Database of Systematic Reviews, 90 from Cochrane Central, 27 from Scopus). Detailed description of the selection process flow is provided in the Figure 1. Forty-two full-text articles have been assessed for eligibility, and only 5 have been considered relevant for this review and included in the qualitative synthesis.9,14–17 Risk of bias assessment is depicted in Figure 2.
Of the 5 studies included in the systematic review, only one was multicentered,9 while the remaining four were single centered.14–17 Two studies were randomized controlled trials,14,17 while the others were cross-over randomized trials.9,15,16 The median [interquartile range] of the mean duration of the studies was 19 [14–22] months. All studies were performed in a university hospital, two (40%) in Italy,9,15 one (20%) in China,14 one (20%) in New Zealand16 and one (20%) in South Korea.17
Characteristics of the population enrolledOverall, 198 exacerbated COPD patients were enrolled by the 5 included studies, with a median (IQR) of 30 [19–65] patients per study. The median (IQR) of the mean patients age receiving HFNC was 72.5 [70.8–75.4] years. In three studies 53 out of 88 patients (60%) were male, while two studies did not report patient gender.14,16 Patient severity was reported as Simplified Acute Physiology Score II (SAPS II) in the study by Di Mussi et al. (39.6 ± 13.2)15 and Longhini et al. (31.5 ± 6.2),9 as Sepsis-related Organ Failure Assessment (SOFA) score by Di Mussi et al. (5.6 ± 2.5)15 and as Acute Physiology and Chronic Health Evaluation (APACHE) score by Jing et al. (11.8 ± 3.1)14; two studies did not report any patient severity score.16,17
HFNC settingsHFNC was used in one study as an alternative treatment to COT in exacerbated COPD patients with need for oxygen,16 in one study as an alternative treatment to NIV in exacerbated COPD patients with mild-to-moderate respiratory acidosis (7.25 < pH < 7.35),17 in two studies after extubation as an alternative to COT15 or NIV,14 and in one study as alternative to COT at NIV interruption in exacerbated COPD patients.9
The median (IQR) of the initial flow delivered with HFNC was 35 [28–50] L/min; in all studies but one, the gas flow was titrated up to the highest flow compatible with patient comfort (maximum allowed between 50–60 L/min). In all studies, the FiO2 was titrated to achieve peripheral oxygen saturation (SpO2) target between 88 and 94%.
Gas exchangeAll studies reported data regarding modifications of gas exchange.
Pilcher et al. measured the transcutaneous carbon dioxide tension (PtCO2) after 30 min of HFNC use, adjusted for the measurement at time zero.16 Compared to COT, HFNC slightly, though significantly, reduced the PtCO2 after 30 min in 24 exacerbated COPD patients with the need for oxygen (mean difference (MD) −1.4 mmHg; 95% Confidence Interval (CI): −2.2 to −0.6; p = 0.001).16
On the other hand, the remaining four studies did not observe any difference in PaCO2.9,14,15,17
In 14 exacerbated COPD patients receiving HFNC and COT after extubation, Di Mussi et al. reported that PaCO2 was 49.9 ± 11.9 and 50.1 ± 12.6 mmHg during the first and second HFNC trial, respectively, while 51.8 ± 12.7 with COT (p value not significant, although not reported).15
Lee et al. randomized 88 exacerbated COPD patients with mild-to-moderate respiratory acidosis to receive or HFNC or NIV.17 After 6 and 24 h, PaCO2 was 46.8 ± 15.2 and 47.0 ± 16.0 mmHg in the HFNC group, while 51.7 ± 17.2 and 49.6 ± 13.7 mmHg in NIV group (p = 0.160 and p = 0.422, respectively).17
Jing et al. randomized 42 exacerbated COPD after extubation to receive or HFNC or NIV.14 PaCO2 was not different between HFNC and NIV at 3 (54.2 ± 9.4 vs. 55.9 ± 11.4 mmHg), 24 (54.7 ± 4.7 vs. 58.9 ± 12.7 mmHg) and 48 h (56.9 ± 10.0 vs. 61.5 ± 16.3 mmHg) (p = 0.780 for all comparisons).14
In the end, our group recently published a cross-over randomized study comparing HFNC with COT and NIV in 30 exacerbated COPD patients ready for an attempt to NIV interruption.9 No PaCO2 differences were reported between three NIV trials (54.1 [47.1–66.4]; 54.8 [46.9–65.0]; 53.3 [46.0–62.3] mmHg), HFNC (52.6 [47.7–63.3] mmHg) and COT (55.0 [48.4–65.4] mmHg) (p = 0.153).9
Oxygenation was also assessed in all studies, through arterial partial pressure of oxygen (PaO2) in 4 studies,9,14,15,17 while with peripheral oxygen saturation (SpO2) in the study by Pilcher et al.16
Three studies did not report differences in oxygenation between HFNC and COT16 or between HFNC and NIV.14,17 Longhini et al. reported that median PaO2/FiO2 was higher during NIV trials (306 [264–358], 303 [271–387] and 309 [270–362] mmHg), compared to both COT and HFNC (p ≤ 0.001 for all comparisons), without significant differences between HFNC (286 [237–318] mmHg) and COT (279 [248–319] mmHg; p = 0.853).9 Di Mussi et al. reported that, to achieve similar PaO2 (∼75 mmHg), COT required higher applied FiO2 (0.80 ± 0.19), as compared to HFNC (0.46 ± 10).15 It should be mentioned, however, that the FiO2 delivered during COT was almost certainly overestimated, as recognized also by the authors.15
Respiratory rateThe medians (IQR) of the mean of respiratory rate were 21.4 [20.2–22.3] breaths/min during HFNC, 21.4 [21.4–24.7] breaths/min during COT and 21.7 [21.0–22.6] breaths/min during NIV. Four studies (80%) did not report differences in respiratory rate between HFNC and COT15,16 or NIV.14,17
In particular, Pilcher et al. reported only a trend towards significant reduction of the respiratory rate (adjusted for the measurement at baseline) at 30 min during HFNC, as opposed to COT (MD −2.0 breaths/min; 95% CI: −4.5 to 0.4; p = 0.099).16 Di Mussi et al. also reported no difference in respiratory rates between HFNC trials (20.5 ± 2.9 and 20.0 ± 1.9 breaths/min) and COT (21.4 ± 4 breaths/min).15
In keeping with the findings by Pilcher and Di Mussi,15,16 Lee et al. did not find differences in terms of median (IQR) respiratory rate between HFNC (24 [20–28] breaths/min) and NIV (24 [22–29] breaths/min; p = 0.235).17 Also in the study by Jing et al., respiratory rate was not different between HFNC and NIV at 3 (21.8 ± 4.9 vs. 21.0 ± 6.1 breaths/min), 24 (21.8 ± 3.8 vs. 22.6 ± 4.7 breaths/min) and 48 h (22.4 ± 4.4 vs. 21.0 ± 4.5 breaths/min) (p = 0.611 for all comparisons).14
Contrary to this, Longhini et al. reported that respiratory rate was greater with COT (24 (20–30) breaths/min), compared with HFOT (20 [18–25] breaths/min; p < 0.001) and NIV trials (21 [18–26], 21 [18–28] and 20 [18–27] breaths/min; p < 0.01 for all comparisons), while not different between HFNC and NIV (p > 0.500).9
Work of breathingWork of breathing was analyzed in two studies only.9,15
Di Mussi et al. recorded the Electrical Activity of the Diaphragm (EAdi) through a dedicated catheter to assess the patients’ respiratory drive and effort.15 The neuroventilatory drive and the work of breathing significantly increased by ∼50% during COT, as compared to HFNC.15 Similarly, Longhini et al. also reported that the diaphragm activation, as assessed by diaphragm ultrasound, increased at NIV interruption by a similar extent with COT, while not during HFNC.9
ComfortPatient comfort was reported in three studies.9,14,16
Pilcher et al. administered at the end of the study protocol a tolerability questionnaire to evaluate both HFNC and COT on a five-point scale for level of comfort, heaviness of nasal interface, noisiness, dryness of nasal passages and ease of breathing. HFNC was reported to be noisier than COT, with a weak evidence for reduced nasal dryness. No differences were found with respect to level of comfort, heaviness of nasal interface and ease of breathing.16
As opposed to findings by Pilcher et al.,16 Jing et al. reported better comfort during HFNC, as opposed to NIV (p = 0.024).14 Interestingly, four patients receiving NIV showed skin breakdowns due to mask pressure sores, while none did in the HFNC group (p = 0.04).14 In keeping with the findings by Jing,14 Longhini et al. also reported that HFNC was better tolerated as compared to both COT (p = 0.004) and NIV (p < 0.001).9
Clinical outcomesThree studies reported some comparisons between HFNC and COT/NIV with respect to any clinical outcomes.9,14,17 Such results derived from secondary studies endpoints in limited sample of patients and, therefore, they should be considered preliminary and requiring further confirmation.
Lee et al. did not find any difference with respect to the intubation rate at day 30 between HFNC (25%) and NIV (27.3%, p = 0.857) and 30-day mortality (15.9% and 18.2%, p = 0.845). HFNC and NIV median durations were 7.0 [5–10] and 8.0 [6–10] days, respectively (p = 0.978).17
Jing et al. did not reported differences between HFNC and NIV with respect to reintubation rate (p = 0.930), duration of invasive mechanical ventilation (p = 0.450), ICU length of stay (p = 0.410) and 28 days-mortality (p = 0.850). Worth noting, HFNC significantly reduced the need for bronchoscopy within 48 h after extubation from 45% to 9.1% of patients (p = 0.008).14
Longhini et al. reported that 36.7% patients failed NIV withdrawal. By chance, at the end of the study protocol, 15 patients received COT and 15 HFOT. Within the following 48 h, NIV was reinstituted in 46.7% of patients receiving COT, while only in 26.7% of those receiving HFNC.9
DiscussionThis systematic review of the literature shows that HFNC has been used, with different aims, to exacerbated COPD patients as an alternative both to COT and NIV. In this setting, HFNC is able to keep PaCO2 unmodified, while oxygenation slightly deteriorates as opposed to NIV. Furthermore, work of breathing is reduced with HFNC by a similar extent to NIV, while it increases by 40–50% during COT. HFNC is also reported to be more comfortable than COT and NIV.
Despite these results, little and limited evidence for improved clinical outcomes is currently available.
In 2014, Okuda et al. firstly reported the use of HFNC in a 73 years-old female patient admitted for COPD exacerbation with the indication to NIV.18 At the improvement of gas exchange and neurological status, NIV was refused by the patient because of poor tolerance. The physician attempted the use of HFNC achieving further gas exchange improvement.
Afterwards, few more exacerbated COPD patients were reported to be successfully treated with HFNC, after NIV failure for poor tolerance and patient refusal.19–21
NIV failure may occur in up to 64% of exacerbated COPD patients, and invasive mechanical ventilation needs to be instituted,3 which may be complicated by a large spectrum of lower respiratory tract infections and septic complications,22 prolong the ICU length of stay and augment mortality.2
Thus, strategies to prevent discomfort and NIV failure should be implemented to reduce potentially severe complications.23
First of all, the choice of a comfortable interface, with limited air-leaks and without excessive tightening of mask straps, is crucial for NIV success.24,25 The use of alternative interfaces, such as the helmet, has been proposed to improve the patient’s comfort and tolerance in different settings.26,27 However, the helmet was initially characterized by a poor patient-ventilator interaction and pressurization performance,27 limiting its use in exacerbated COPD patients.28 A new generation helmet, specifically designed to overcome such drawbacks, has been successfully tested in exacerbated COPD patients.29,30
Patient-ventilator interaction and synchrony is another determinant for NIV success.31,32 Although the occurrence of asynchronous events can be partially managed by optimizing the ventilator settings32 or using innovative modes of ventilation, such as Neurally Adjusted Ventilatory Assist (NAVA),24,27,30,33,34 it remains difficult to recognize such events by the sole ventilator waveform observation without the use of additional signals.35
It should be mentioned that NIV is mainly used in exacerbated COPD patients to improve gas exchange and to reduce the work of breathing.2 Preliminary data suggest that HFNC is not inferior to NIV in achieving such goals. This review highlights the fact that HFNC reduces work of breathing to a similar extent as NIV,9 while keeping similar PaCO2 values.9,14,15,17 Worth noting, HFNC has the advantage of being more comfortable than NIV,9,14 because it does not require a “true” interaction or synchrony between the system and the patient.
In view of these considerations and the physiological mechanisms highlighted above, HFNC can play a role in the integrated management interventions for patients with COPD exacerbation.
First, HFNC might have a role as an alternative to NIV in exacerbated COPD patients in cases of mild-to-moderate respiratory acidosis (i.e., 7.25 < pH < 7.35). In a small randomized controlled trial including 88 exacerbated COPD patients, Lee et al. showed similar CO2 clearance at 6 and 24 h of treatment, intubation rate and 30-day mortality between HFNC and NIV.17 In keeping with Lee et al.,17 a recent prospective observational study, involving 30 patients with mild-to-moderate hypercapnic ARF, also showed that HFNC was effective in normalizing gas exchange with an acceptable rate of no responders, requiring NIV.36
To date, a multicenter randomized controlled study to evaluate if HFNC is not-inferior to NIV in reducing PaCO2, in patients with an exacerbation of COPD and mild-to-moderate respiratory acidosis, is ongoing and will provide us with relevant clinical information regarding the efficiency of HFNC treatment (ClinicalTrials.gov; NCT03370666).
Second, HFNC might also be used as an alternative to NIV14 or COT,15 immediately after extubation, to prevent post-extubation respiratory failure. In up to 23% of patients after planned extubation, ARF may occur within 48–72 h.37 However, the application of NIV soon after extubation was shown to reduce the incidence of post-extubation respiratory failure and 90-days mortality in hypercapnic patients.37 Preliminary data have shown that HFNC was not inferior to NIV after extubation in COPD patients, with respect to gas exchange and vital parameters.14 Furthermore, as opposed to COT, HFNC significantly reduced the work of breathing by ∼50% after extubation.15
Third, HFNC might be a valid alternative to COT during breaks of NIV or to facilitate NIV discontinuation. In COPD patients with hypercapnic ARF, the rate of NIV discontinuation failure may be relatively high, due to the inability to sustain spontaneous breathing over time. According to the findings of Longhini et al.,9 HFNC should in principle be preferred to COT for NIV withdrawal or during intervals between NIV periods. A randomized controlled trial is currently ongoing to ascertain in patients with hypercapnic ARF whether HFNC, as compared to COT, may increase the number of ventilator-free days and living patients at day 28, when applied at NIV discontinuation.38
Finally, HFNC might also be used in acute exacerbated hypercapnic and dyspnoic COPD patients without respiratory acidosis, as an alternative to COT.
In relation to this, Pilcher et al. have shown some physiological benefits of HFNC, as compared to COT, mainly limited to slight improvement of gas exchange.16 Unfortunately, work of breathing was not assessed, although its reduction could be expected during HFNC, based on some physiological rationale.5
ConclusionsAlthough the available clinical data for application of HFNC in COPD exacerbation are increasing over time, there are still some unanswered questions regarding practical aspects of its use.
Randomized controlled trials are needed to clarify the real efficacy of HFNC and the best target population during COPD exacerbation. In addition, data should confirm whether therapy with HFNC could play a role in addition to or as an alternative to COT and NIV in the various settings described above.