To assess the efficacy and safety of high-flow nasal cannula (HFNC) in elderly patients with acute respiratory failure (ARF) not due to COVID-19, refractory to treatment with conventional oxygen therapy and/or intolerant to noninvasive ventilation (NIV) or continuous positive airway pressure (CPAP) and without criteria for admission to intensive care units (ICU).
MethodsProspective observational study of patients with ARF treated with HFNC who presented clinical and arterial blood gas deterioration after 24 h of medical treatment and oxygenation by conventional systems. The degree of dyspnoea, gas exchange parameters (arterial O2 pressure/inspired O2 fraction ratio (PaO2/FiO2); oxygen saturation measured by oximetry/ inspired fraction of oxygen (Sp02/Fi02), ROX index), degree of patient tolerance and mortality were evaluated. These were measured at discharge from the emergency department (ED), 24 h after treatment with conventional oxygenation and 60, 120 min and 24 h after initiation of HFNC. The results were analyzed for all patients as a whole and for patients with hypercapnia (arterial carbon dioxide tension (PaCO2) < 45 mmHg) separately.
Results200 patients were included in the study between November 2019 and November 2020, with a mean age of 83 years, predominantly women (61.9%), obese (Body Mass Index (BMI) 31.1), with high comorbidity (Charlson index 4) and mild-moderate degree of dependence (Barthel 60). A number of 128 patients (64%) were hypercapnic. None had respiratory acidosis (pH 7.39). Evaluation at 60 min, 120 min and 24 h showed significant improvement in all patients and in the subgroup of hypercapnic patients with respect to baseline parameters in respiratory rate (RR), dyspnoea, ROX index, PaO2/FiO2, SpO2/FiO2 and patient comfort. No changes in PaCO2 or level of consciousness were observed. HFNC was well tolerated. Ten patients (5%) died due to progression of the disease causing ARF.
ConclusionsHFNC is an effective and safe alternative in elderly patients with ARF not due to COVID-19, refractory to treatment with conventional oxygen therapy and/or intolerant to NIV or CPAP and without criteria for admission to ICU.
Acute respiratory failure (ARF) in older adults is a frequent reason for consultation in the emergency department (ED) and often accompanies exacerbations of chronic pulmonary and cardiac diseases.1,2 ARF may present with severity criteria that warrant admission to the intensive care unit (ICU). However, factors such as age over 80 years, the severity of associated comorbidity, the functional capacity of the patient and the willingness of the patient and family, may contribute to the decision to limit the therapeutic effort in these patients.3
Among the non-invasive respiratory support therapies (NIRS), high-flow nasal cannula therapy (HFNC) is recognized as the first therapeutic option in patients with hypoxemic ARF.4–6 Its mechanism of action is considered broad-spectrum, and there is evidence of its efficacy also in patients with acute hypercapnic respiratory failure.7,8
There is a group of elderly (>75 years) and very elderly (>90 years) patients with ARF who, after receiving medical treatment and conventional oxygen therapy, remains with dyspnoea and refractory hypoxemia. These patients are mostly admitted outside ICU and generally present acute exacerbation of chronic heart failure (CHF), acute pulmonary edema, acute exacerbation of chronic obstructive pulmonary disease (AE-COPD) or pneumonia. They frequently manifest intolerance to non-invasive mechanical ventilation (NIV) or continuous positive airway pressure (CPAP), and present limitations when it comes to staging treatment towards invasive mechanical ventilation.9
It is in this specific group of patients that HFNC can play a very important role, since it offers a real and effective alternative in different situations, fundamentally in hypoxemic but also in moderate hypercapnic ARF when the patient does not tolerate NIV or CPAP and the only possibility we have is to treat them outside ICU.10–12 Most studies have not included a strictly elderly population,13 and those with large series of patients over 75 years old treated outside the ICU for ARF are scarce.14 We present a study which aimed to evaluate the efficacy and safety of HFNC in elderly patients with ARF of any origin excluding COVID-19, without criteria for admission to critical care units and refractory to treatment with conventional oxygen therapy and/or intolerant to NIV or CPAP, who were admitted to a hospital area specialized in NIRS dependent on the ED.
MethodsProspective observational study with consecutive inclusion of patients older than 75 years, that presented ARF not due to COVID-19 and were treated with HFNC in a hospital ward specialized in NIRS dependent on the ED, during the period November 2019-November 2020.
Patients with ARF admitted to the ED who presented clinical deterioration, no improvement in dyspnoea, tachypnea with respiratory rate (RR) greater than 25 bpm or hypoxemia with arterial O2 pressure /inspired O2 fraction ratio (Pa02/Fi02) less than 300, after 24 h of medical treatment and oxygenation by conventional systems (Venturi mask with a Kendall® type humidification system from COVIDIEN® laboratories without heating, with a flowmeter up to 15 l/min) with a Fi02 ≥ 35%, were included.
These patients were started on HFNC (AIRVO2® System with OPTIFLOW࣪ cannulas, Fisher & Paykel, New Zealand) with a flow between 40 and 60 lpm and the minimum Fi02 to maintain a peripheral oxygen saturation measured by oximetry (Sp02) of 94-96% in hypoxemic patients and 88-92% in hypercapnic patients with associated chronic cardiac or pulmonary diseases.
Patients with hemodynamic instability defined by a systolic blood pressure (BP) less than 100 mmHg, and those with indication for NIV showing a pH less than 7.25, use of accessory musculature or abdominal paradoxical breathing were excluded. We did not exclude patients with hypercapnia (PaCO2 > 45 mmHg) with early intolerance (first 120 min) to NIV and those with an express order or desire not to perform orotracheal intubation.
The study was conducted in accordance with the 2010 Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects, and the patients or their immediate family members gave their consent to participate. The study was approved by the Hospital Clinical Research (ISABIAL) and Ethical Committee.
The variables collected were: dyspnoea (measured with the modified Borg scale15), RR, heart rate (HR), BP, Sp02, Pa02, arterial carbon dioxide tension (PaC02), bicarbonate, lactate, pH, Fi02, Pa02/Fi02 and oxygen saturation measured by oximetry/ inspired fraction of oxygen (Sp02/Fi02), ROX index (SpO2:FiO2 / RR)16 and HFNC settings (flow in lpm). These measurements were taken at 24 h after treatment with conventional oxygenation (basal values) and at 60, 120 min and 24 h after initiation of HFNC. The results were analyzed for all patients as a whole and for patients with hypercapnia (PaCO2 > 45 mmHg) separately. To evaluate safety, we recorded the degree of patient comfort at the same times using a visual analog scale (VAS) where 0 is no comfort and 10 is maximum comfort.17 Technique failure was considered as the need for NIV. Mortality during admission, at one week and one month after discharge, readmission at one week and one month after discharge, and secondary adverse events produced by the technique, were also registered.
Baseline patient data included demographic data (age and sex) and comorbidity (history of hypertension, diabetes, dyslipidemia, ischemic heart disease, valvular heart disease, atrial fibrillation, cerebrovascular disease, COPD, asthma, malignancies and diagnosis of dementia). Comorbidity was assessed using the Charlson index,18 the degree of dependence for basic activities of daily living using the Barthel index19 and the use of home oxygen therapy.
The data analyzed related to the episode of ARF included: systolic BP, RR HR, Sp02, modified Borg dyspnoea scale and comfort scale. The following analytical parameters were collected: hemoglobin, creatinine, estimated glomerular filtration rate, sodium, ultrasensitive troponin T and natriuretic peptides (pro-BNP), as well as data from chest X-ray and chest ultrasound if available.
Statistical analysisFor the descriptive analysis, absolute and relative frequencies were used for qualitative variables, and mean with standard deviation or median and interquartile range for quantitative variables. To evaluate the influence of HFNC at predetermined times on the different variables studied, a one-factor ANOVA was used or the nonparametric Wilcoxon test if normality criteria were not met, which was contrasted by the Kolmogorov-Smirnov test. The degree of significance was set at 0.05 and the statistical analysis was performed using the SPSS v.24 computer package.
ResultsIn the period from November 2019 to November 2020, a total of 200 patients were included in the study, with a mean age of 83 years, predominantly women (61.9%), obese (Body Mass Index (BMI) 31.1), with high comorbidity (Charlson index 4) and mild-moderate degree of dependence (Barthel 60). A number of 128 patients (64%) were hypercapnic. None of them had respiratory acidosis (pH 7.39). The baseline characteristics of the patients are shown in Table 1.
Baseline characteristics of the 200 patients with acute and acute on chronic respiratory failure.
Data presented as median (interquartile range) or n (%).
COPD: Chronic obstructive pulmonary disease. BMI: Body Mass Index. IQR: Interquartile range.
At inclusion, patients were tachypneic (RR: 28.5 bpm), with significant dyspnoea (Borg: 8) and a good level of consciousness (Glasgow: 15). They had mild respiratory distress (PaO2/FiO2: 217) and slight hypercapnia (PaCO2 48 mmHg), without respiratory acidosis (pH 7.40).
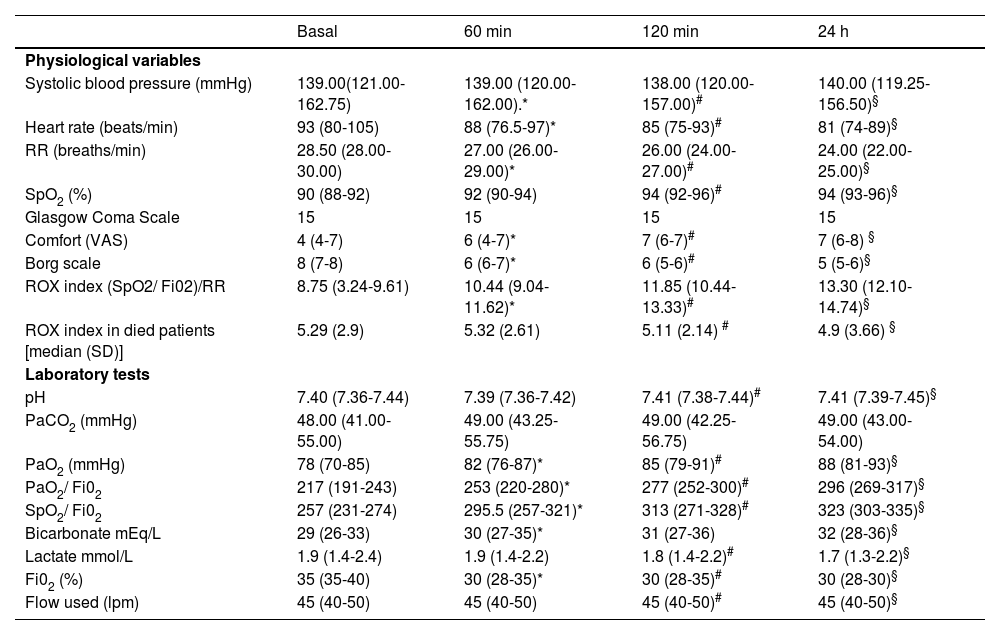
Evaluation at 60 min, 120 min and 24 h showed a significant improvement over baseline parameters in RR, dyspnoea, ROX index, PaO2/FiO2, SpO2/FiO2 and patient comfort. No changes were seen in PaCO2 (48 vs 49 mmHg at 24 h) or level of consciousness (Glasgow 15 vs 15). Table 2 shows the clinical and blood gas evolution data of the 200 patients.
Clinical and gasometric evolution of the 200 patients treated with high-flow nasal cannula therapy.
| Basal | 60 min | 120 min | 24 h | |
|---|---|---|---|---|
| Physiological variables | ||||
| Systolic blood pressure (mmHg) | 139.00(121.00-162.75) | 139.00 (120.00-162.00).* | 138.00 (120.00-157.00)# | 140.00 (119.25-156.50)§ |
| Heart rate (beats/min) | 93 (80-105) | 88 (76.5-97)* | 85 (75-93)# | 81 (74-89)§ |
| RR (breaths/min) | 28.50 (28.00-30.00) | 27.00 (26.00-29.00)* | 26.00 (24.00-27.00)# | 24.00 (22.00-25.00)§ |
| SpO2 (%) | 90 (88-92) | 92 (90-94) | 94 (92-96)# | 94 (93-96)§ |
| Glasgow Coma Scale | 15 | 15 | 15 | 15 |
| Comfort (VAS) | 4 (4-7) | 6 (4-7)* | 7 (6-7)# | 7 (6-8) § |
| Borg scale | 8 (7-8) | 6 (6-7)* | 6 (5-6)# | 5 (5-6)§ |
| ROX index (SpO2/ Fi02)/RR | 8.75 (3.24-9.61) | 10.44 (9.04-11.62)* | 11.85 (10.44-13.33)# | 13.30 (12.10-14.74)§ |
| ROX index in died patients [median (SD)] | 5.29 (2.9) | 5.32 (2.61) | 5.11 (2.14) # | 4.9 (3.66) § |
| Laboratory tests | ||||
| pH | 7.40 (7.36-7.44) | 7.39 (7.36-7.42) | 7.41 (7.38-7.44)# | 7.41 (7.39-7.45)§ |
| PaCO2 (mmHg) | 48.00 (41.00-55.00) | 49.00 (43.25-55.75) | 49.00 (42.25-56.75) | 49.00 (43.00-54.00) |
| PaO2 (mmHg) | 78 (70-85) | 82 (76-87)* | 85 (79-91)# | 88 (81-93)§ |
| PaO2/ Fi02 | 217 (191-243) | 253 (220-280)* | 277 (252-300)# | 296 (269-317)§ |
| SpO2/ Fi02 | 257 (231-274) | 295.5 (257-321)* | 313 (271-328)# | 323 (303-335)§ |
| Bicarbonate mEq/L | 29 (26-33) | 30 (27-35)* | 31 (27-36) | 32 (28-36)§ |
| Lactate mmol/L | 1.9 (1.4-2.4) | 1.9 (1.4-2.2) | 1.8 (1.4-2.2)# | 1.7 (1.3-2.2)§ |
| Fi02 (%) | 35 (35-40) | 30 (28-35)* | 30 (28-35)# | 30 (28-30)§ |
| Flow used (lpm) | 45 (40-50) | 45 (40-50) | 45 (40-50)# | 45 (40-50)§ |
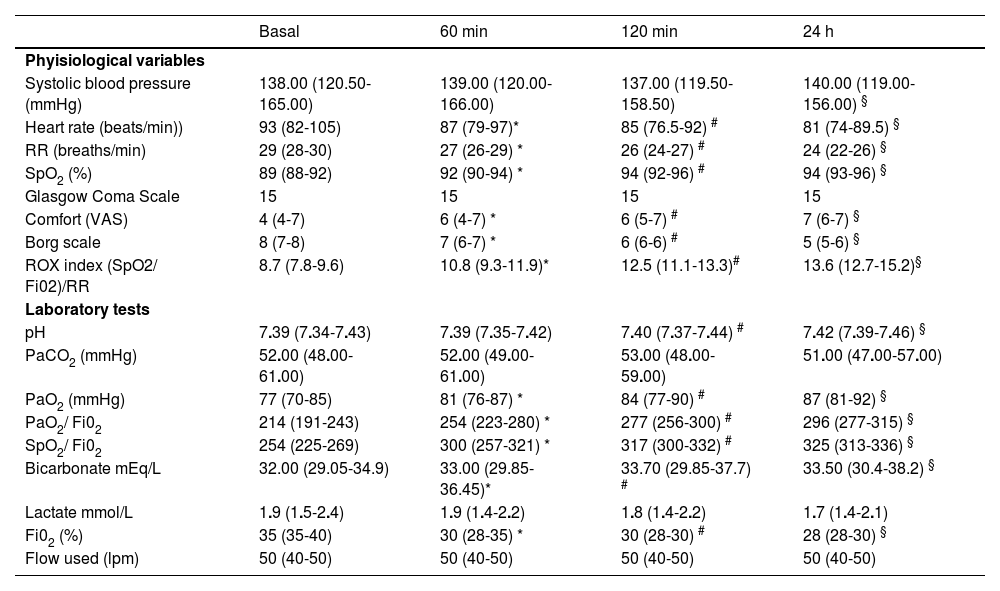
Independent analysis of the 128 hypercapnic patients also showed a significant improvement in the assessment at 60 min, 120 min and 24 h with respect to baseline parameters in RR, dyspnoea, ROX index, PaO2/FiO2, SpO2/FiO2 and patient comfort. There was no significant change in PaCO2 (52 vs 51 at 24 h) or deterioration in the level of consciousness (Glasgow 15 vs 15). Table 3 shows the clinical and blood gas evolution of the 128 patients.
Clinical and blood gas evolution of 128 patients with PaCO2 > 45 mmHg treated with high-flow nasal cannula therapy.
| Basal | 60 min | 120 min | 24 h | |
|---|---|---|---|---|
| Phyisiological variables | ||||
| Systolic blood pressure (mmHg) | 138.00 (120.50-165.00) | 139.00 (120.00-166.00) | 137.00 (119.50-158.50) | 140.00 (119.00-156.00) § |
| Heart rate (beats/min)) | 93 (82-105) | 87 (79-97)* | 85 (76.5-92) # | 81 (74-89.5) § |
| RR (breaths/min) | 29 (28-30) | 27 (26-29) * | 26 (24-27) # | 24 (22-26) § |
| SpO2 (%) | 89 (88-92) | 92 (90-94) * | 94 (92-96) # | 94 (93-96) § |
| Glasgow Coma Scale | 15 | 15 | 15 | 15 |
| Comfort (VAS) | 4 (4-7) | 6 (4-7) * | 6 (5-7) # | 7 (6-7) § |
| Borg scale | 8 (7-8) | 7 (6-7) * | 6 (6-6) # | 5 (5-6) § |
| ROX index (SpO2/ Fi02)/RR | 8.7 (7.8-9.6) | 10.8 (9.3-11.9)* | 12.5 (11.1-13.3)# | 13.6 (12.7-15.2)§ |
| Laboratory tests | ||||
| pH | 7.39 (7.34-7.43) | 7.39 (7.35-7.42) | 7.40 (7.37-7.44) # | 7.42 (7.39-7.46) § |
| PaCO2 (mmHg) | 52.00 (48.00-61.00) | 52.00 (49.00-61.00) | 53.00 (48.00-59.00) | 51.00 (47.00-57.00) |
| PaO2 (mmHg) | 77 (70-85) | 81 (76-87) * | 84 (77-90) # | 87 (81-92) § |
| PaO2/ Fi02 | 214 (191-243) | 254 (223-280) * | 277 (256-300) # | 296 (277-315) § |
| SpO2/ Fi02 | 254 (225-269) | 300 (257-321) * | 317 (300-332) # | 325 (313-336) § |
| Bicarbonate mEq/L | 32.00 (29.05-34.9) | 33.00 (29.85-36.45)* | 33.70 (29.85-37.7) # | 33.50 (30.4-38.2) § |
| Lactate mmol/L | 1.9 (1.5-2.4) | 1.9 (1.4-2.2) | 1.8 (1.4-2.2) | 1.7 (1.4-2.1) |
| Fi02 (%) | 35 (35-40) | 30 (28-35) * | 30 (28-30) # | 28 (28-30) § |
| Flow used (lpm) | 50 (40-50) | 50 (40-50) | 50 (40-50) | 50 (40-50) |
HFNC was well tolerated, with patients showing a significant improvement in the comfort VAS. The most frequent side effects observed were heat intolerance (37.5%), flow discomfort (19%), headache (17%) and a feeling of self-limited chest tightness (17%). These did not lead to discontinuation of the therapy in any of the cases.
A total of 10 patients died (5%), with progression of the triggering cause of ARF being the main cause of death. All belonged to the NIV intolerant group and had a non-intubation (DNI) order. The mean ROX index of these patients, in all periods studied, was lower and/or did not progress sufficiently well when compared to the rest of the patients.
Analyzing the 200 patients together, 7 patients (3.5%) were readmitted after 7 days and 17 (8.5%) after 30 days. In the group of hypercapnic patients, 3 patients (2.34%) were readmitted at 7 days and 8 patients (6.25%) were readmitted at 30 days. The main cause for readmission was AE-COPD and acute exacerbation of CHF.
DiscussionHFNC has been used for years in the treatment of patients with hypoxemic ARF, mainly in the ICU setting, with its use in conventional hospital wards being more anecdotal, a situation that changed during the pandemic caused by the SARS-CoV-2 virus.20 It has been demonstrated that in patients with hypoxemic ARF, HFNC exerts multiple physiological effects including less inspiratory effort and improved lung volume and compliance.7,21,22 HFNC also decreases breathing frequency and work of breathing and reduces the need for respiratory support escalation.22 Moreover, HFNC decreases anatomical dead space and generates a positive airway pressure, enhancing patient comfort.23
A question of great importance is related with the role of HFNC concerning the effect on transpulmonary pressure. It is well known that increases in dynamic transpulmonary pressure may cause patient self-induced lung injury.24 Grieco et al.25 compared HFNC and helmet NIV in patients with hypoxic ARF. Helmet NIV improved oxygenation, reduced dyspnoea, inspiratory effort, and simplified pressure-time support, with similar transpulmonary pressure swings, PCO2 and comfort. Most interesting is that patients with low inspiratory effort during HFNC showed increases in dynamic transpulmonary driving pressure while on helmet NIV, and this was associated with the need for endotracheal intubation. Schifino et al.26 have analyzed the effects of HFNC, CPAP and NIV on inspiratory effort in moderate-severe COVID-19 patients. NIV was superior to HFNC and CPAP in reducing the inspiratory effort, maintaining transpulmonary pressure similar to the other NIRS. All this data highlight the importance of the careful monitoring when using NIRS to detect and prevent abnormal increases in dynamic transpulmonary driving pressure.
There are few studies in the literature that address the use of HFNC in patients with: ARF not due to COVID-19, who are elderly, in a palliative situation or with a DNI order, and most of these include patients in ICU and resuscitation areas during the weaning period of invasive mechanical ventilation.27–31
Our study analyzes the efficacy and safety of the use of HFNC in 200 patients over 75 years of age with ARF, who have a poor response to conventional oxygen therapy or intolerance to NIV in patients with a DNI order. These criteria have been applied generally in COVID-19 patients, but there are no studies in elderly non-COVID-19 patients treated with HFNC outside an ICU with a number of patients such as the one we present. We have independently analyzed those with moderate hypercapnia, and it should be pointed out that none of them had respiratory acidosis.
Our series is characterized by a predominance of women, who are obese, with high comorbidity and hypercapnic respiratory failure. The most frequent cause of ARF in our series was acute exacerbation of CHF, coinciding with the results of the comparative study of HFNC versus conventional oxygen therapy in an ED by Rittayamai et al.32 in a younger population (64.6 years old). We believe that the use of HFNC in patients with acute exacerbation of CHF without acute pulmonary edema is underestimated by clinicians, and that there is an important group of these patients with ARF who could benefit from its early use.33 De novo ARF due to pneumonia and AE-COPD with hypoxemia and moderate hypercapnia were frequent causes (second and third respectively) of ARF, data similar to those from other studies carried out in conventional hospitalization units.34,35 In hypercapnic patients, acute exacerbation of CHF and EACOPD were also the most frequent causes of ARF, which confirms the high comorbidity among patients over 75 years of age. This association is very frequent among patients who consult the ED for dyspnoea, with a high percentage of admissions and revisits after discharge.32,36,37
In our series we observed a significant improvement in oxygenation (PaO2/FiO2 ratio, SpO2/FiO2 ratio), RR and dyspnoea measured with the Borg scale. This improvement is evident at as early as 60 min, but is preferentially manifested 24 h after initiation of therapy. These data are in line with those of recent reviews on the use of HFNC in patients with ARF.38,39
The use of HFNC in patients with hypercapnic ARF has been the subject of controversy in the past. The positive effects that HFNC could exert on hypercapnia, both in the upper airway (optimization of respiratory mucosal biology, effective washout of CO2 during expiration and reduction of dead space) and lower airway (positive end-expiratory pressure effect -PEEP-, alveolar recruitment, increase in expiratory tidal volume), have been demonstrated both in pulmonary simulators and at the bedside.40,41
In the analysis of the 128 patients with hypercapnia, we found a significant improvement in all the periods analyzed in dyspnoea, RR, and oxygenation parameters PaO2/FiO2 and Sp02/FiO2, coinciding with other studies carried out with patients with or without hypercapnia.42,43 There is a tendency towards improvement, although not significant, in PaCO2 and pH. These data reaffirm the possible use of HFNC in patients with moderate hypercapnia without respiratory acidosis, always using low Fi02 to achieve an SpO2 of 88-92% and with high flows (up to 60 lpm).44
Our protocol was designed to start on HFNC with a flow between 40 and 60 lpm and the minimum Fi02 to maintain a Sp02 of 94-96% in hypoxemic patients and 88-92% in hypercapnic patients. However, the SpO2 at 120 min and 24 h in hypercapnic patients was 94%, slightly above the predefined target. This divergence could be explained by several reasons; firstly, the need for a higher FiO2 due to desaturation episodes, which should have been regulated according to the objectives once stabilized. Secondly, and most important, the study ended in November 2020, so it was influenced during 6 months by the COVID pandemic, mainly the shortage of healthcare professionals and the less attention to the kind of patient typically attended in the unit (patient over 75 years old, pluripathological, with a non-intubation order and whose admission is refused in other units). Nevertheless, the hypercapnic patients receiving HFNC did not show any clinical deterioration related to a higher SpO2.
One of the main characteristics of HFNC compared to other NIRS and conventional oxygen therapy is the degree of comfort and tolerance shown by patients. Our results confirm this data (significant improvement in the comfort analog scale 24 h after initiation of therapy), as has been observed in other publications.45,46
Regarding the side effects observed in our patients, they were of little relevance and did not lead to the failure of the technique in any case, which is in line with what has been observed in other studies.47 Although HFNC is considered a simple technique, correct monitoring and training are essential for correct patient management.
In-hospital mortality was 5% (10 patients) and its main cause was the progression of the triggering cause of ARF. In these patients, the ROX index showed a significant deterioration at 120 min and 24 h, a value that coincides with that obtained by Lee et al.48 at 2 and 6 h after initiation of HFNC, immediately before extubation in an ICU. We believe that the use of the ROX index is fundamental when monitoring patients treated with HFNC49 as has been confirmed in the current pandemic situation due to SARS CoV-2 infection.50 The 10 patients who died in our series belonged to the group of patients intolerant to NIV with a DNI order.
Our work has several limitations. The first is the absence of a control group and randomization, assuming the weaknesses of a descriptive observational study. Secondly, the study was carried out in a hospital area specialized in NIRS dependent on the ED, which could imply a bias as there are no similar units in other hospitals in our country. This unit works as a respiratory intermediate care unit. Thirdly, the study period was affected by the first waves of the COVID-19 pandemic (March-November 2020), which may imply a selection bias of non-COVID-19 patients who presented ARF and were admitted to the hospital. Finally, although both hypoxemic and hypercapnic patients were included, none presented respiratory acidosis, which does not allow us to extrapolate our results to patients with impaired pH during exacerbation.
In summary, we present a descriptive observational study showing the efficacy, safety and comfort of a series of 200 patients over 75 years of age with ARF not due to COVID-19, treated with HFCN outside the intensive care unit. This is the study with the largest number of patients with these characteristics published to date and shows that the use of HFCN in selected patients may be an alternative for elderly patients with ARF who remain hypoxemic and in respiratory distress after 24 h of conventional treatment. Randomized studies are needed to confirm these data and to provide higher quality evidence for the use of HFCN in the elderly population.
Authors declare not to have any conflict of interest related to the content of this manuscript.