Late onset Pompe disease (LOPD) is a glycogen storage disorder characterised by progressive skeletal and respiratory (i.e. diaphragmatic) muscle weakness and ventilatory pump failure (VPF).1 Enzyme replacement therapy (ERT) can prolong survival.2 Noninvasive ventilation (NIV) is often used with low bi-level positive airway pressure (PAP) which may be inadequate for optimal respiratory muscle rest and ventilator support. Higher pressure support (PS) of 14 cm H2O or more often called Noninvasive ventilatory support (NVS) needs to be used. While NIV can improve gas exchange, sleep, and quality of life (QOL) for mild cases, only increased pressures can be used for continuous support to prolong survival.3 Both NIV and NVS can be provided during sleep via different interfaces. The nasal mask or mouth-piece is better during daytime. Intermittent abdominal pressure ventilator (IAPV) can also provide daytime support. It consists of an air-sack inside a corset inflated by a ventilator. The sack compresses the abdomen to raise the diaphragm and to increase tidal volumes.4,5 IAPV use by LOPD patients has not yet been described, and we aimed to report its feasibility in this study.
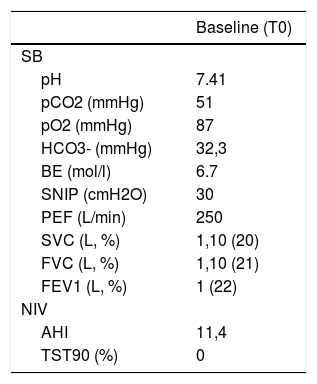
Case Study: A 22-year-old male university student, diagnosed with LOPD at age two, began bi-weekly ERT at age 11. The ERT was adjusted over the years based on his body weight. At age 13 he began nocturnal CPAP for obstructive sleep apnoea (OSA). Within two years, due to hypercapnia, he was transitioned to NIV, and since then continued with limited follow-up. Settings: inspiratory (I)PAP 12, expiratory (E)PAP 4 cm H2O, respiratory rate 14/min which he presented to us using in 2019. During our first visit, he had severe scoliosis, emaciation (BMI 12.8 kg/m2), and recent onset of hyper-somnolence and morning headaches. Pulmonary function testing (PFT), arterial blood gases (ABG), and polysomnography (PSG) using NIV data are in Table 1. His QoL was assessed by the McGill Questionnaire.6 Over the previous 30 days mean nocturnal use was 9 h with average Vt 350 mL with 85% of the breaths triggered. Then, IPAP was gradually increased to 16 (i.e. PS of 12 cm H2O).2 This resulted in 600 mL tidal volumes which he was happy to continue.
Baseline characteristics.
| Baseline (T0) | |
|---|---|
| SB | |
| pH | 7.41 |
| pCO2 (mmHg) | 51 |
| pO2 (mmHg) | 87 |
| HCO3- (mmHg) | 32,3 |
| BE (mol/l) | 6.7 |
| SNIP (cmH2O) | 30 |
| PEF (L/min) | 250 |
| SVC (L, %) | 1,10 (20) |
| FVC (L, %) | 1,10 (21) |
| FEV1 (L, %) | 1 (22) |
| NIV | |
| AHI | 11,4 |
| TST90 (%) | 0 |
SB, spontaneous breathing; BE, base excess; SNIP, sniff nasal inspiratory pressure; PEF, peak expiratory flow; SVC, slow vital capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; NIV, Non-invasive ventilation; AHI, apnea-hypopnea index; TST90, total sleep time with oxyhemoglobin saturation below 90%.
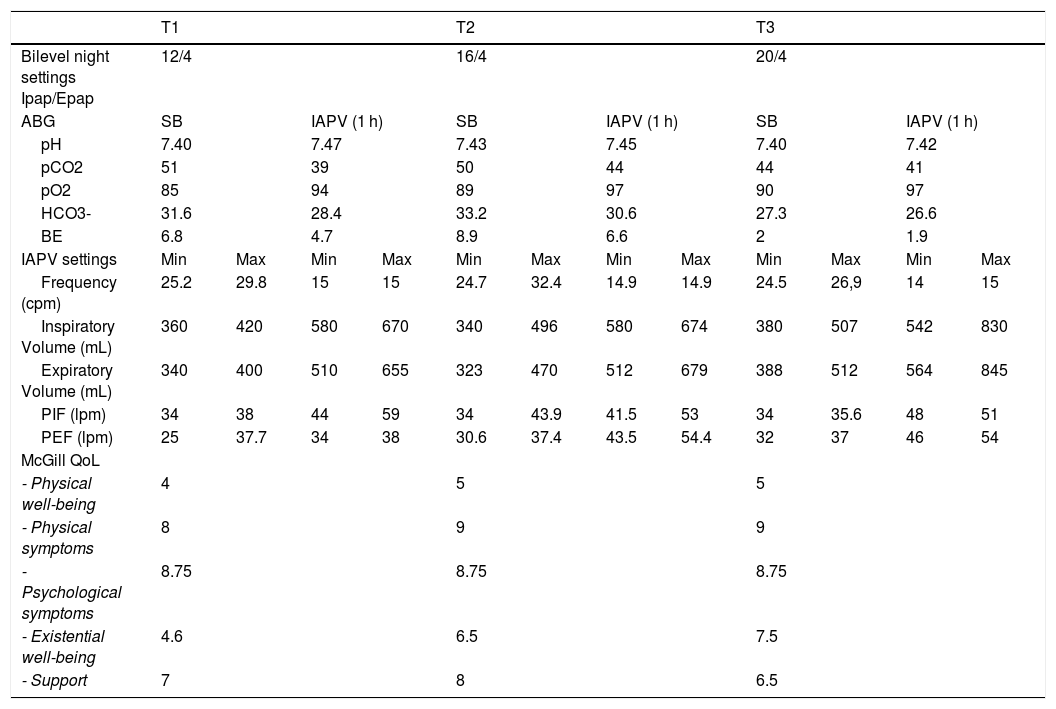
One month later, he continued 12 cm PS NIV for a mean 10 h per night, had an apnea-hypopnea index of 0.7, and average Vt 500 mL with 96% of breaths triggered. However, daytime dyspnea and fatigue continued, and despite a trial with the same ventilator he was not inclined to use mouthpiece, or NIV via nasal mask at university or socially. Diurnal hypercapnia persisted so the IAPV was introduced with a belt pressure (PBelt) of 50 cm H2O (LUNA DS, Dima Italia Inc., Bologna, Italy). His symptoms cleared, ABG, and tidal volumes improved (Table 2). Transcutaneous CO2 (TCO2) averaged 45 mmHg during IAPV use and 51 mmHg during nocturnal NIV. He was instructed to increase PS to NVS settings of IPAP 18, EPAP 4 cm H2O. Delivered volumes increased up to 750 mL. He also combined used the IAPV about 4 h during daytime.
Settings of the two respiratory support ventilators, and data results.
| T1 | T2 | T3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bilevel night settings Ipap/Epap | 12/4 | 16/4 | 20/4 | |||||||||
| ABG | SB | IAPV (1 h) | SB | IAPV (1 h) | SB | IAPV (1 h) | ||||||
| pH | 7.40 | 7.47 | 7.43 | 7.45 | 7.40 | 7.42 | ||||||
| pCO2 | 51 | 39 | 50 | 44 | 44 | 41 | ||||||
| pO2 | 85 | 94 | 89 | 97 | 90 | 97 | ||||||
| HCO3- | 31.6 | 28.4 | 33.2 | 30.6 | 27.3 | 26.6 | ||||||
| BE | 6.8 | 4.7 | 8.9 | 6.6 | 2 | 1.9 | ||||||
| IAPV settings | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max |
| Frequency (cpm) | 25.2 | 29.8 | 15 | 15 | 24.7 | 32.4 | 14.9 | 14.9 | 24.5 | 26,9 | 14 | 15 |
| Inspiratory Volume (mL) | 360 | 420 | 580 | 670 | 340 | 496 | 580 | 674 | 380 | 507 | 542 | 830 |
| Expiratory Volume (mL) | 340 | 400 | 510 | 655 | 323 | 470 | 512 | 679 | 388 | 512 | 564 | 845 |
| PIF (lpm) | 34 | 38 | 44 | 59 | 34 | 43.9 | 41.5 | 53 | 34 | 35.6 | 48 | 51 |
| PEF (lpm) | 25 | 37.7 | 34 | 38 | 30.6 | 37.4 | 43.5 | 54.4 | 32 | 37 | 46 | 54 |
| McGill QoL | ||||||||||||
| - Physical well-being | 4 | 5 | 5 | |||||||||
| - Physical symptoms | 8 | 9 | 9 | |||||||||
| - Psychological symptoms | 8.75 | 8.75 | 8.75 | |||||||||
| - Existential well-being | 4.6 | 6.5 | 7.5 | |||||||||
| - Support | 7 | 8 | 6.5 | |||||||||
Legend: T1, 2, 3, Time 1, 2, 3 of follow up. ABG, ambient air arterial blood gas analysis; SB, spontaneous breathing; IAPV, intermittent abdominal pressure ventilator; PIF, Peak inspiratory Flow; PEF, Peak inspiratory Flow; QoL, quality of life.
At 3 month follow-up he reported reduction of morning headaches and sleepiness. The ABG and QoL improved, while PFT and PSG remained stable. Given the residual hypercapnia we further increased IPAP to 20 cm H2O. Six 6 months later (T3), 24-h mean TCO2 was 38.9 during IAPV, and 44 mmHg during NVS, and ABG and QOL further improved (Table 2).
This case demonstrates both diurnal and nocturnal blood gas improvements by increasing typical NIV settings to NVS settings. However, with advancing weakness, diurnal hypercapnia can persist or return. For this, IAPV use was practical and effective for this LOPD patient who was not compliant with diurnal NVS. Indeed, NVS has become the cornerstone of daytime support for VPF. It normalizes ABG, and continuous dependence on it has prolonged the lives of some post-polio patients by over 66 years, Duchenne muscular dystrophy patients by 30 years, and spinal muscular atrophy type 1 by 25 years so far, without resort to tracheotomy.3 Varying interfaces helps avoid excessive skin pressure, mouth and upper airway dryness, mucus impaction, social interaction and eating difficulties.3,5,7 Daytime IAPV facilitates activities of daily living to improve QoL, especially for patients unwilling to use daytime NVS.4
There are several advantages to the IAPV: no facial interfaces, increased cough flows and tidal volumes, improved speech duration, and it is simple to don and wear. Disadvantages include: regurgitation after meals, need to be seated, and cannot be used in a bath or shower.4,5
In conclusion, in this young LOPD patient IAPV normalized alveolar ventilation, tidal volumes, gas exchange, and relieved daytime symptoms to improve QoL in both his "physical and existential well-being". It also avoided NVS side effects for the combined IAPV use. Further studies are warranted to broaden its application.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank Dr L. Macchia and Dr C. Santomasi for their substantial contribution to this study.