There are currently no established markers for aspiration pneumonia. We hypothesized that bronchoalveolar lavage (BAL) amylase and pepsin might be candidate biomarkers for aspiration pneumonia.
MethodsThis cross-sectional study reviewed consenting adults who underwent clinically-indicated bronchoscopy at Aizu Medical Center. BAL samples were obtained using standardized methods. Amylase levels were measured in our clinical laboratory, and pepsin levels were assessed by ELISA.
ResultsAspiration pneumonia was clinically diagnosed based on the guidelines of the Japanese Respiratory Society in 48 of the 327 participants. Median BAL salivary amylase and pepsin levels in this group were 702.0 U/L and 12.7 ng/ml respectively, which were significantly higher than in non-aspiration pneumonia patients. BAL amylase ≥204 U/L had 77.1% sensitivity and 84.2% specificity as a diagnostic index, and the area under the receiver operating characteristic (ROC) curve was 0.859 (95% confidence interval (CI), 0.803-0.915). Similarly, BAL pepsin levels of ≥7.45 ng/ml had 87.2% sensitivity and 59.9% specificity for identifying aspiration, and the area under the ROC curve was 0.757 (95% CI, 0.688-0.826). Multivariate regression demonstrated that BAL amylase ≥204 U/L and BAL pepsin ≥7.45 ng/ml were associated with significantly higher odds for aspiration pneumonia (odds ratio (OR) 10.0, 95% CI, 4.51-22.2, and OR 8.81 95% CI, 3.32-23.4, respectively). There were no significant associations between risk factors for aspiration pneumonia and BAL amylase and pepsin levels.
ConclusionBAL amylase and pepsin might be useful biomarkers for suggesting aspiration pneumonia, and could be objective markers without relying on known risk factors for aspiration.
Aspiration pneumonia (AP) occurs at any age, but it is more common in the elderly. Although AP is commonly thought of as hospital-acquired pneumonia (HAP), it is also an important cause of community-acquired pneumonia (CAP). Reportedly, 5 to 15% of cases of CAP are AP.1 Through multicenter research in Japan, Teramoto et al. reported that more than 60% of hospitalized CAPs in the elderly were AP.2 Aspiration of small amounts of oropharyngeal secretions during sleep is called “microaspiration”, and large volume aspiration is termed “macroaspiration”.3 With a unique technique using indium111 chloride pasted on the gums, Kikuchi and his colleague demonstrated that microaspiration plays a pivotal role in the development of CAP in the elderly.4 In their study, scanning of the thorax demonstrated microaspiration in 71% of CAP patients and only 10% of control subjects.4
AP is typically a clinical diagnosis, and there are currently no diagnostic criteria for AP.3 The Japanese Respiratory Society (JRS) defines AP in the guidelines for management of pneumonia in adults (Figure S1).5 According to their classification, a case with proven aspiration is a “definite” case. Cases with dysphagia, such as those with central nervous system diseases, dementia due to cerebrovascular disorders or degenerative diseases such as Alzheimer's disease or on antipsychotic medication for delirium management6 are “probable” cases. While macroaspiration of gastric content can be relatively easily detected, there are also many “suspected” cases of AP in clinical practice. Microaspiration, occult pulmonary aspiration, is pivotal when treating cases “suspected” of AP.
Some researchers suggested the possibility of diagnosis of microaspiration by assessing bronchoalveolar lavage (BAL) pepsin levels.7 According to these studies, the leading mechanism for microaspiration is gastroesophageal reflux disease (GERD).8 However, BAL-pepsin only reflects gastrointestinal fluid aspiration.9
On the other hand, Weiss et al. demonstrated that BAL-amylase is associated with risk factors for aspiration in mechanically-ventilated patients.10 Salivary amylase is produced by salivary glands, particularly the parotid.11 It is rare for amylase to be produced in tracheal or bronchoalveolar cells, except in amylase-producing small cell lung carcinoma cases.12 Therefore, detection of amylase in the lower respiratory tract suggests dysfunction of the swallowing reflex, which might have diagnostic value in proving aspiration due to dripping of oral secretions. Different studies have shown the utility of tracheal amylase to diagnose microaspiration in mechanically-ventilated patients,13 and as a marker of chronic pulmonary aspiration in children with chronic respiratory illness.11
Here, we investigated the potential of elevated BAL-amylase levels as a diagnostic indicator of AP compared to BAL-pepsin levels. We also evaluated the association between elevated biomarkers and risk factors for AP.
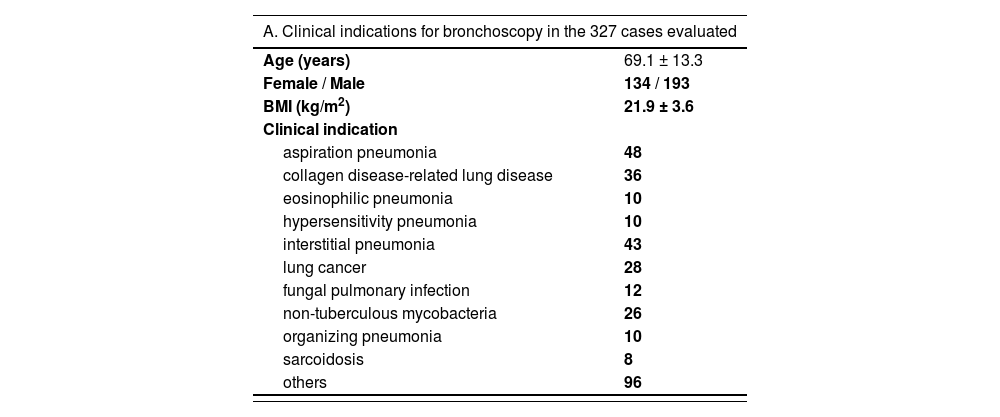
MethodsPatient selection and study designWe conducted a cross-sectional study of adult patients undergoing clinically-indicated bronchoscopy at Aizu Medical Center from June 2014 to May 2020. This study included 327 consenting patients, among whom AP was confirmed in 48 cases (Table 1A) by three experienced pulmonologists and two experienced radiologists based on adult pneumonia guidelines by the JRS.5
A: Clinical indications for bronchoscopy in the 327 cases evaluated. B: Characteristics of patients with aspiration pneumonia (48 cases). There was overlap in underlying diseases. Data represent mean ± standard deviation. BMI, body mass index; NHCAP, nursing and healthcare associated pneumonia; HAP, hospital acquired pneumonia; CAP, community acquired pneumonia; GERD, gastric esophageal reflux disease; COPD, chronic obstructive pulmonary disease; BA, bronchial asthma; DM, diabetes mellitus; BI, Brinkman index. C: Serum and bronchoalveolar lavage (BAL) fluid data in the 48 aspiration pneumonia cases
Data are presented as mean ± standard deviation and median [interquartile range]. WBC, white blood cell; CRP, C-reactive protein; S-amylase, salivary amylase; P/F, Partial pressure of arterial oxygen (PaO2) / fraction of inspired oxygen (FiO2) ratio; BALF, bronchoalveolar lavage fluid.
This study protocol was approved by the Independent Ethics Committee of Fukushima Medical University for conducting this research (Approval No. 29063). All patients provided written informed consent before participation. The study was registered in UMIN Clinical Trials Registry: UMIN 000043587.
Data collectionClinical data collected included: age, sex, body mass index (BMI), underlying diseases, especially those considered risk factors for aspiration, oral medicines, smoking history, blood tests such as white blood cells, c-reactive protein, salivary amylase, and arterial blood gas analysis, and BAL fluid (BALF).
Bronchoscopy was performed for various clinical indications, including AP, interstitial pneumonia, lung cancer, non-tuberculous mycobacteria (NTM), and sarcoidosis. All procedures were performed under conscious sedation by two pulmonologists with 30 years of clinical experience. BAL was performed in the subsegmental bronchus with the abnormal lesion by instillation of 100-150 ml of buffered saline divided into 2-3 aliquots. BAL-amylase and its isozymes were measured at our clinical laboratory, and BAL-pepsin was analyzed using an ELISA kit for pepsin (Cloud-Clone Corp, TX, USA).14
Statistical analysisData were expressed as the mean ± standard deviation or the median with 25-75% quartile range (QR). The F test was performed to compare mean values in the two groups. Student's t-test and Welch's t-test were used to compare values with and without homoscedasticity, respectively. One-way ANOVA was used for multiple comparisons. The Mann-Whitney U-test and Wilcoxon signed-rank test were used when comparing the medians of independent and dependent variables, respectively. Kruskal-Wallis test was used for multiple comparisons. Receiver-operating characteristic (ROC) curve analysis was used to evaluate the clinical validity of BAL-amylase and BAL-pepsin, and the Chi-square test was used to examine their association with risk factors for AP. Univariate and multivariate logistic regression analyses were performed to investigate the relationship between BAL-amylase or BAL-pepsin and AP. The results are expressed as odds ratio (OR)s with 95% confidence interval (CI)s. All analyses were performed using EZR and BellCurve for Excel.
ResultsCharacteristics of patients with aspiration pneumoniaForty-eight patients (15 females and 33 males) with aspiration pneumonia were included in the study. HAP was identified in three patients who were not bedridden, and the remaining 45 cases were CAP. Average patient age was 74.5 ± 8.2 years, significantly older than that in the overall cohort, and BMI was 19.7 ± 3.7 kg/m2, which was significantly lower than in the entire cohort (Table 1B). Medical history and underlying diseases that were risk factors for aspiration were listed, and the number of AP patients with the various risk factors for aspiration is shown in Table 1B. Among them, 18 cases did not have any predisposing risk factors.
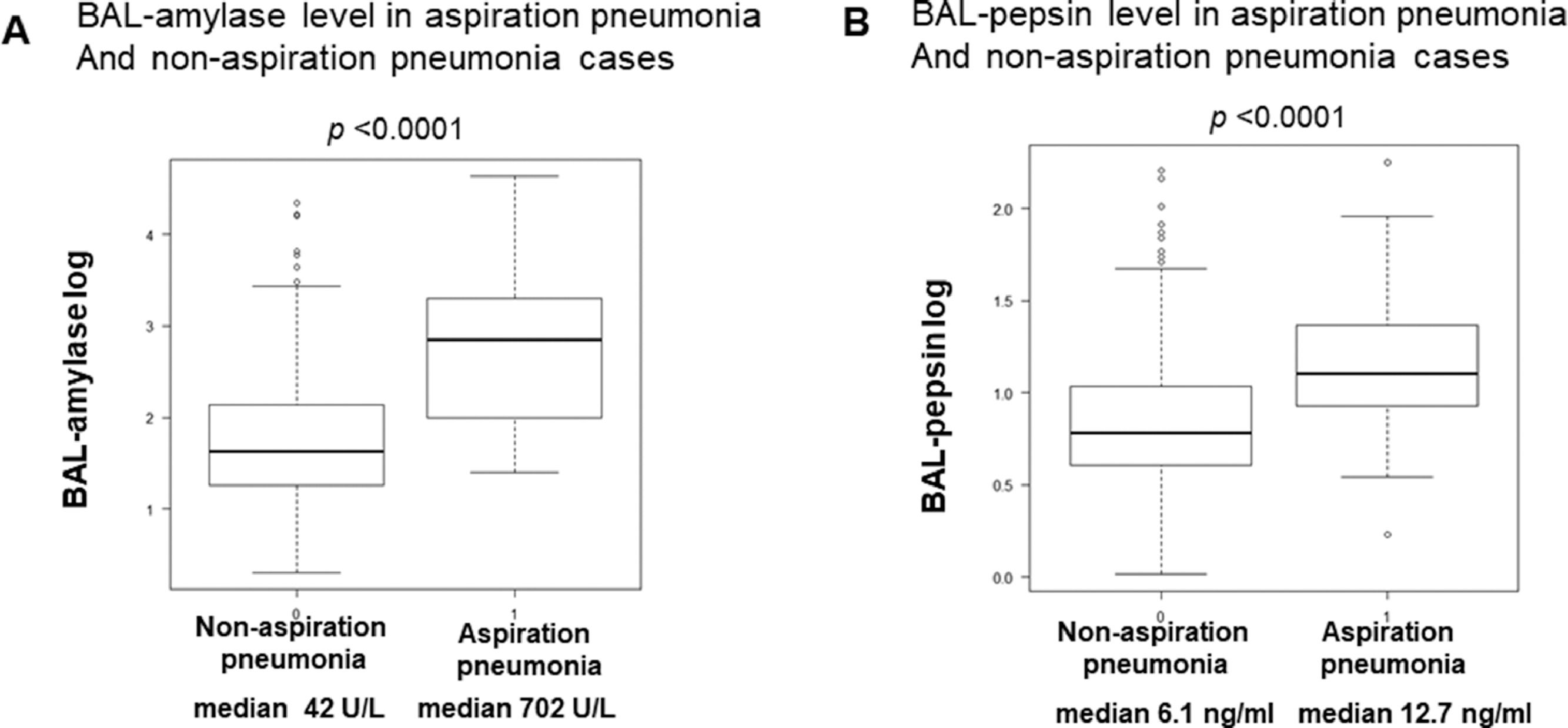
Serum and BALF data in aspiration pneumonia patientsTable 1C shows serum and BAL fluid laboratory data in AP patients. Neutrophil-dominated inflammation was detected in serum. The average partial pressure of arterial oxygen (PaO2) / fraction of inspired oxygen (FiO2), ratio (P/F) ratio was 348 ± 68, which was not low, and there were no cases of severe hypoxia. The ratio of neutrophils to macrophages and lymphocytes in BAL fluid was higher than normal BAL fractions.15 The median value of BAL salivary amylase with a probability of 98-100% and BAL-pepsin levels in AP patients were 702.0 U/L and 12.7 ng/ml, respectively, significantly higher than their levels in non-AP cases. Serum amylase levels were not elevated (Fig. 1A, B).
A: BAL-amylase levels in aspiration pneumonia and non-aspiration pneumonia cases. Boxplot (median [interquartile range]) of BAL-amylase levels (units/L) in non-aspiration and aspiration pneumonia cases. The vertical axis shows the log scale of BAL-amylase levels. B: BAL-pepsin levels in aspiration pneumonia and non-aspiration pneumonia cases. Boxplot (median [interquartile range]) of BAL-pepsin levels (ng/ml) in non-aspiration and aspiration pneumonia cases. The vertical axis shows the log scale of BAL-pepsin levels.
Median BAL-amylase levels were significantly higher in AP cases versus other conditions such as interstitial pneumonia, lung cancer, NTM, and sarcoidosis (Figure S2A). Median BAL-pepsin levels also showed a similar tendency (Figure S2B). Thus, BAL-amylase and pepsin levels were significantly higher in AP than in other diseases.
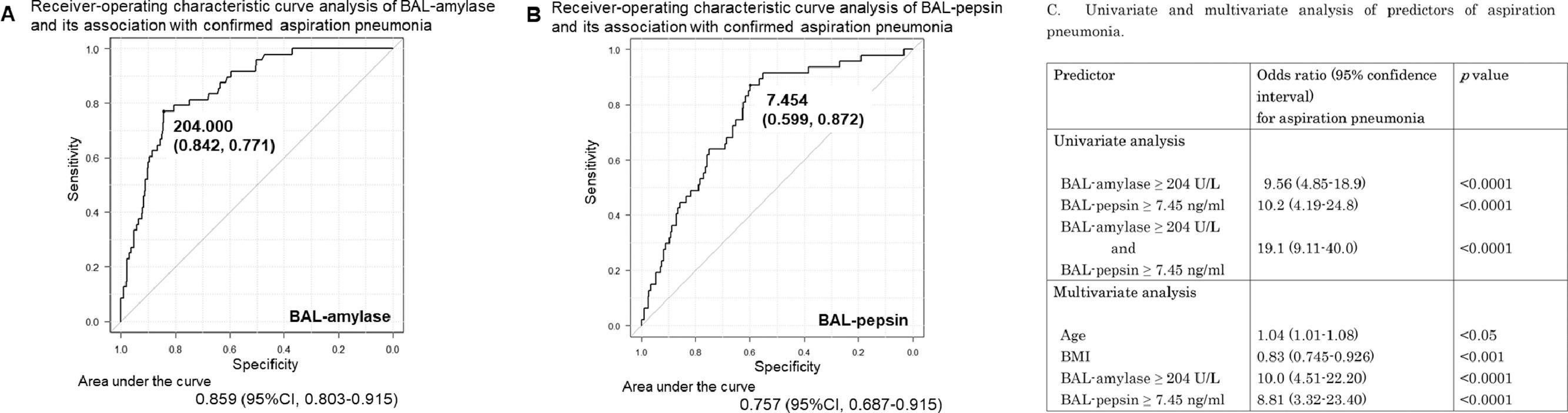
Clinical validity of BAL-amylase and BAL-pepsin for diagnosing aspiration pneumoniaROC analysis demonstrated that BAL-amylase at a cut-off value of 204 U/L differentiates between patients with AP and other conditions with a sensitivity of 77.1% and specificity of 84.2%. The area under the ROC curve was statistically significant, at 0.85 (95% CI 0.80-0.91) (Fig. 2A). On the other hand, ROC analysis of BAL-pepsin at a cut-off value of 7.45 ng/ml had a sensitivity of 87.2% and specificity of 59.9% for differentiating AP, and the area under the ROC curve was also statistically significant, at 0.76 (95% CI 0.69-0.83) (Fig. 2B).
A, B: Receiver-operating characteristic (ROC) curve analysis of BAL-amylase and BAL-pepsin and their association with confirmed aspiration pneumonia. A: A cut-off value of BAL-amylase of 204 U/L had a specificity and sensitivity of 0.842 and 0.771, respectively, for identifying aspiration pneumonia. The area under the ROC curve was 0.86 (95% confidence interval (CI) 0.80-0.91). B: The cut-off value of BA-pepsin of 7.45 (ng/ml) had a specificity and sensitivity of 0.599 and 0.872, respectively. The area under the ROC curve was 0.76 (95% CI 0.69-0.83). C: The table showed univariate and multivariate analyses of predictors of aspiration pneumonia.
Univariate regression demonstrated that BAL-amylase of 204 U/L and BAL-pepsin of 7.45 ng/ml were associated with significantly higher odds for AP (OR 9.56, 95% CI 4.9-18.9, p=7.57e-11, and OR 10.2, 95% CI 4.2-24.8, p=3.14e-07, respectively). Multivariate regression also demonstrated similar results and indicated that aging and low BMI might also be related to AP. These data suggest the clinical validity of BAL-amylase and BAL-pepsin assessments for the diagnosis of AP (Fig. 2C).
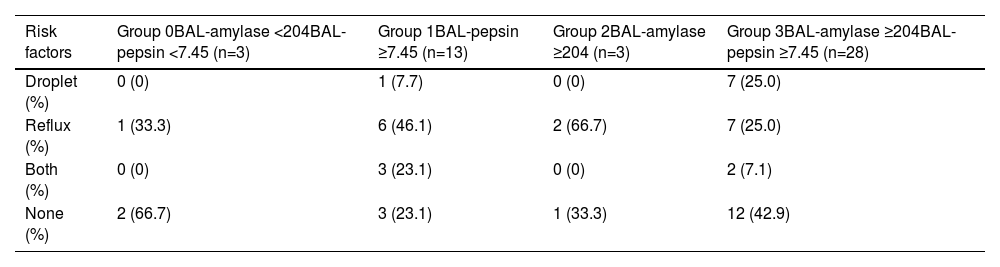
Evaluation of BALF data in aspiration pneumonia patients grouped according to cut-off values of BAL-amylase and BAL-pepsinBased on the results of Fig. 2, AP patients were grouped according to the cut-off values of BAL-amylase and BAL-pepsin of 204.0 U/L and 7.45 ng/ml, respectively, as: Group 0: BAL-amylase < 204.0 U/L group and BAL-pepsin < 7.45 ng/ml: (n=3), Group 1: BAL-pepsin ≥ 7.45 ng/ml (n=13), Group 2: BAL-amylase ≥ 204.0 U/L (n=3), and Group 3: BAL-amylase ≥ 204.0 U/L and BAL-pepsin ≥ 7.45 ng/ml (n=28). Here there was a total of 47 cases excluding one case because it had only BAL amylase data and no BAL pepsin data. BAL-amylase levels in groups 2 and 3 were significantly higher than in the other groups. BAL-pepsin levels were significantly elevated in both groups 1 and 3. Although serum neutrophils were not different among the groups, the median percentage of BAL neutrophils in groups 1 and 3 were 62.7% and 75.5%, respectively, showing a tendency to be higher than in the other groups although the difference was not significant (Table S1).
Risk factors for aspiration and BAL-amylase and BAL-pepsin in aspiration pneumonia casesGERD, esophageal surgery, and gastrectomy were defined as risk factors for “reflux”, and cerebrovascular diseases, dementia, mental illness with psychopharmaceuticals, and alcoholism, which can cause dysphagia, were defined as “droplet” risk factors. Risk factors for AP were investigated in the four groups mentioned above. Notably, high-BAL pepsin level cases in groups 1 and 3 did not necessarily have risk factors for “reflux”, although nearly half of them showed a tendency for “reflux”. High BAL-amylase level cases in groups 2 and 3 also showed that few cases had risk factors for “droplet” (Table 2). As seen in Table 2, the proportion of cases without any risk factors was higher not only in group 0 but also in group 3. There was no significant association between risk factors for AP and levels of BAL-amylase and pepsin.
Relationship between risk factors for aspiration pneumonia and cut-off values in BAL fluid. Patients were grouped as described in Table S1. Risk factors were classified as “Droplet”-related microaspiration due to dripping of oral secretions, “Reflux”-related microaspiration of gastro-intestinal contents, “Both” which included “Droplet” and “Reflux” microaspiration, and “None” with no risk factors for aspiration. Data show the number and percentage of patients in each group. BAL, bronchoalveolar lavage.
We investigated BAL-amylase and BAL-pepsin as potential biomarkers for AP. In this study, both BAL-amylase and BAL-pepsin levels were significantly elevated in AP patients. Ours is the first study to investigate the association between BAL-amylase and adult AP. We found that BAL-amylase levels ≥ 204 U/L could diagnose AP with a sensitivity of 77.1% and specificity of 84.2%, supporting the results of previous studies, even if the different mechanisms between AP and VAP.16 Similarly, BAL-pepsin levels ≥ 7.45 ng/ml have a sensitivity and specificity of 87.2% and 59.9%, respectively, for diagnosing AP. Additionally, BAL-amylase ≥ 204 U/L and BAL pepsin ≥ 7.45 ng/ml were associated with significantly higher odds for AP, with ORs of 9.56 and 10.2, respectively, and the presence of both BAL-amylase ≥ 204 U/L and BAL-pepsin ≥ 7.45 ng/ml had an even higher OR of 19.1. These results suggest the clinical validity of using BAL-amylase and BAL-pepsin levels to diagnose AP, although our study results are limited because this was a cross-sectional study.
AP is a clinical diagnosis, and in addition to macroaspiration, AP due to microaspiration should be suspected in most cases. The initial expectation was that increases in BAL-amylase and BAL-pepsin levels would occur relatively independently, and AP caused by the dripping of oral secretions and pneumonia caused by reflux of gastrointestinal contents might be diagnosed separately. Contrary to our expectations, however, our study showed that 28 of 48 AP cases had higher levels than the cut-off values of both BAL-pepsin and amylase. Normally, microaspiration is prevented by physiological defense mechanisms, such as the swallowing reflex and cough reflex. Therefore, high BAL-amylase levels, reflecting dripping of oral secretions, and high BAL-pepsin levels, reflecting reflux of gastrointestinal contents, might be considered a requirement for diagnosing AP. Furthermore, BAL-amylase might be considered an initial indicator of the collapse of natural defense mechanisms.
It is essential to determine risk factors for swallowing disturbances. In the present study, 18 cases had no underlying risk factors. As shown in Table 2, although group 3 tended to contain more risk factors than group 2, risk factors for aspiration were not significantly associated with BAL-amylase or pepsin levels. There are several possible reasons for this: (1) medical staff did not obtain their medical history accurately; (2) patients did not divulge or did not remember their history of previous diseases; and (3) they might have had undiagnosed asymptomatic risk factors for microaspiration. Hence, risk factors for AP in our cases could have been underestimated. However, even if medical histories related to microaspiration are thoroughly obtained, there is a limit to understanding all the risk factors in patients. Patients do not always understand their symptoms correctly regarding GERD,17 and many cases might not have been accurately diagnosed by endoscopy. Basal ganglia strokes are reportedly associated with microaspiration.18 Asymptomatic undiagnosed cerebral infarction might also lead to dysphagia. Other risk factors, such as low BMI and aging, are reportedly related to microaspiration. As shown in Table 1B, AP cases had significantly lower BMI than other patients, suggesting a decline in muscle mass. Aging-related sarcopenia was previously reported to lead to dysphagia.19 The results of multivariate analysis in our study also supported this fact. Hence, although it is important to accurately understand the risk factors for AP, the development of objective biomarkers of AP is also urgent.
Our study has several limitations. First, this was a cross-sectional study. A well-designed prospective study is needed to clarify the causal relationship between AP and BAL-pepsin and amylase levels. A prospective study might also prove whether elevation of BAL-amylase is an early indicator of microaspiration leading to AP. Secondly, this study was conducted at a single facility, and the study population was naturally limited. A multi-center clinical trial adopting standardized diagnostic criteria or bronchoscopy procedures for AP is needed in the future. Third, our study showed that BAL-amylase and pepsin levels were not associated with risk factors for AP. As mentioned above, our study might have underestimated the risk factors for AP. Risk factors such as GERD and cerebral infarction should be investigated more actively, and swallowing reflex, as an indicator of microaspiration, should also be evaluated by a simple swallowing provocation test.20 Finally, bronchoscopy is an invasive approach performed by specialists and cannot be performed everywhere. Additionally, there is the risk of complications due to the procedure, and careful selection of patients must be made to reduce the risk of complications. Hence, bronchoscopy measuring these biomarkers is not always definitively recommended. It should be considered for cases such as recurrent pneumonia with unclear risk factors or pneumonia of unknown cause which AP is a differential diagnosis to consider. Simpler and less-invasive examinations, such as evaluation of exhaled breath condensate pepsin21 or intratracheal secretions, instead of BAL fluid collection, should be more practical and attempted in the future. As further studies, once BAL-amylase and pepsin are accepted as biomarkers of AP, their association with severity of AP should be proved, and severity index and high-resolution computed tomography image findings should be evaluated in future studies.
ConclusionMeasurement of BAL-amylase and BAL-pepsin levels might contribute to the diagnosis of AP. We found that BAL-amylase ≥ 204 U/L and BAL-pepsin ≥ 7.45 ng/ml are associated with significantly higher odds of AP. Although a cohort study is needed to prove causality, our study suggests that BAL-amylase and pepsin might be potential biomarkers for substantiating a clinical diagnosis of AP.






![A: BAL-amylase levels in aspiration pneumonia and non-aspiration pneumonia cases. Boxplot (median [interquartile range]) of BAL-amylase levels (units/L) in non-aspiration and aspiration pneumonia cases. The vertical axis shows the log scale of BAL-amylase levels. B: BAL-pepsin levels in aspiration pneumonia and non-aspiration pneumonia cases. Boxplot (median [interquartile range]) of BAL-pepsin levels (ng/ml) in non-aspiration and aspiration pneumonia cases. The vertical axis shows the log scale of BAL-pepsin levels. A: BAL-amylase levels in aspiration pneumonia and non-aspiration pneumonia cases. Boxplot (median [interquartile range]) of BAL-amylase levels (units/L) in non-aspiration and aspiration pneumonia cases. The vertical axis shows the log scale of BAL-amylase levels. B: BAL-pepsin levels in aspiration pneumonia and non-aspiration pneumonia cases. Boxplot (median [interquartile range]) of BAL-pepsin levels (ng/ml) in non-aspiration and aspiration pneumonia cases. The vertical axis shows the log scale of BAL-pepsin levels.](https://static.elsevier.es/multimedia/25310437/0000002900000005/v1_202309041851/S2531043722001040/v1_202309041851/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)







