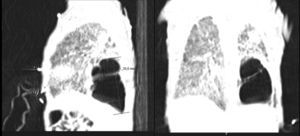
Bronchopulmonary dysplasia (BPD) is a major complication of extreme prematurity. The lungs are characterized by areas of emphysema, and fibrosis.1 Large pneumatoceles due to acquired localized emphysema over-inflation are recognized but are relatively rare in advanced BPD.2–4
A male newborn of 580g birthweight was born at 26 weeks of gestation by c-section to a 33 years old 4G, 3P, gipsy mother, after a full cycle of corticosteroids. The pregnancy was regularly followed, and was complicated by gestational diabetes, preeclampsia and intrauterine growth restriction. The 1st/5th/10th minutes Apgar score were 3/5/7.
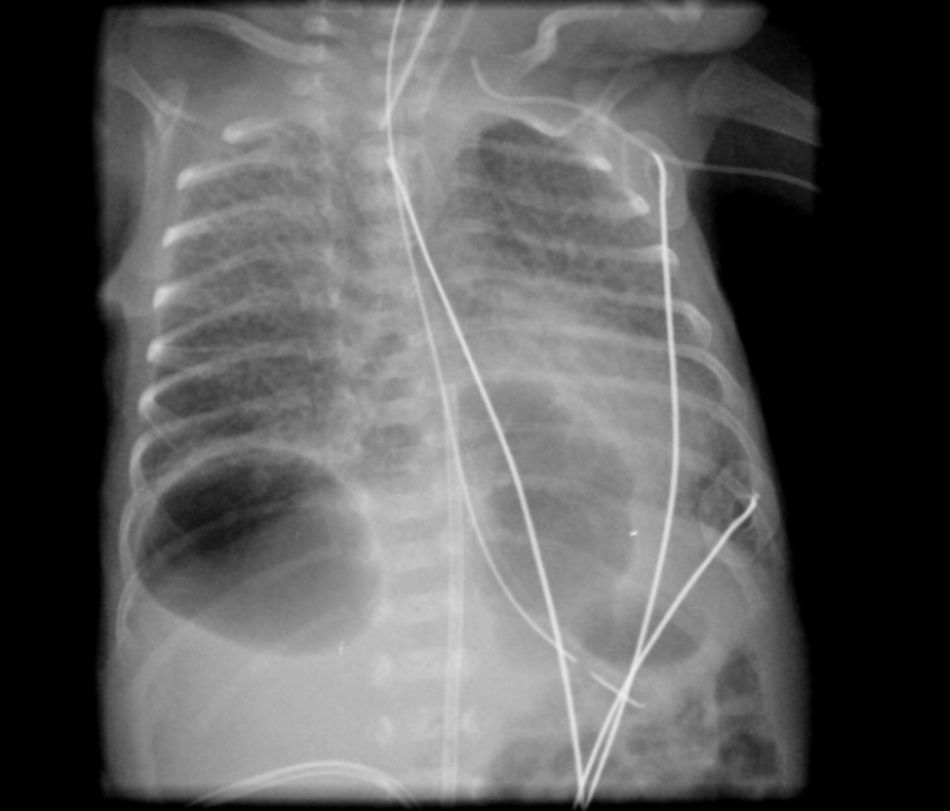
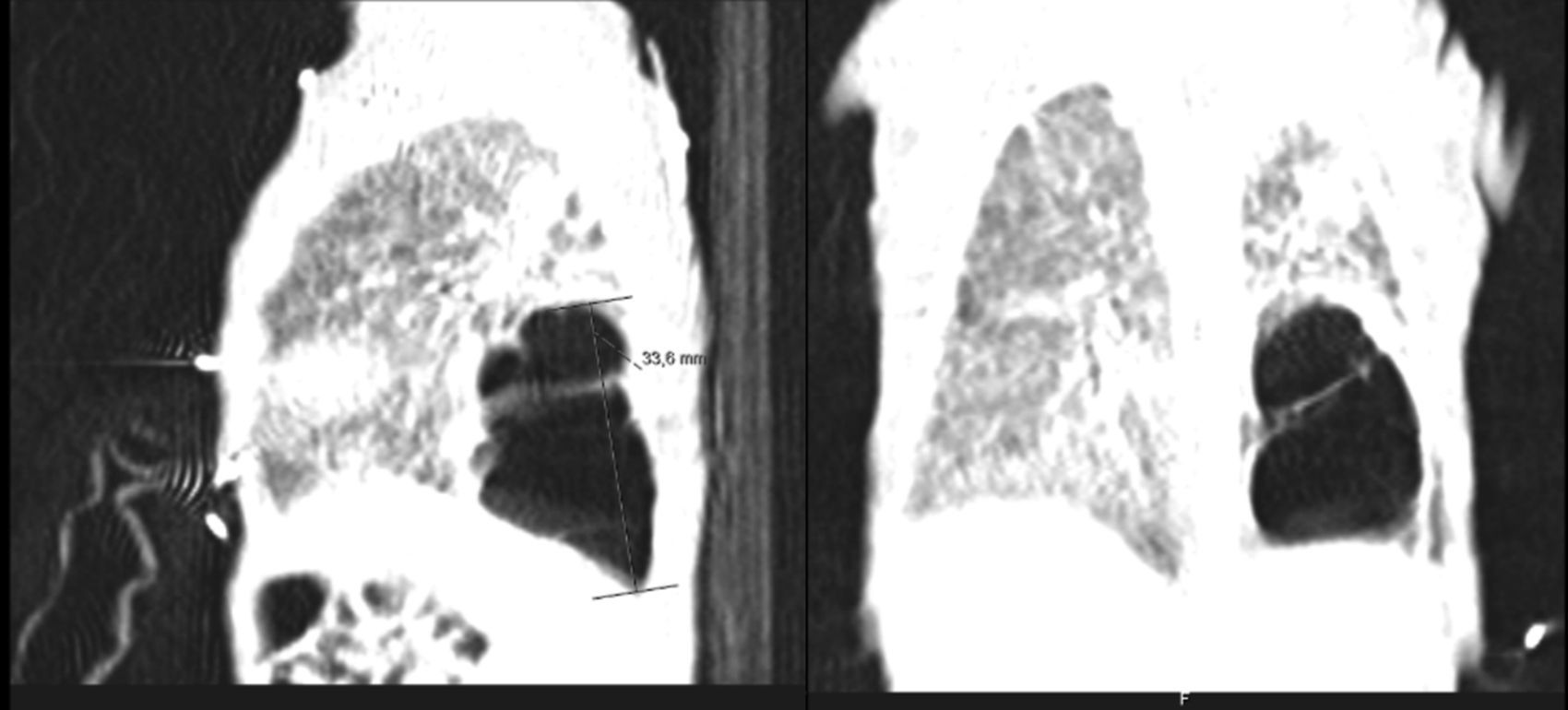
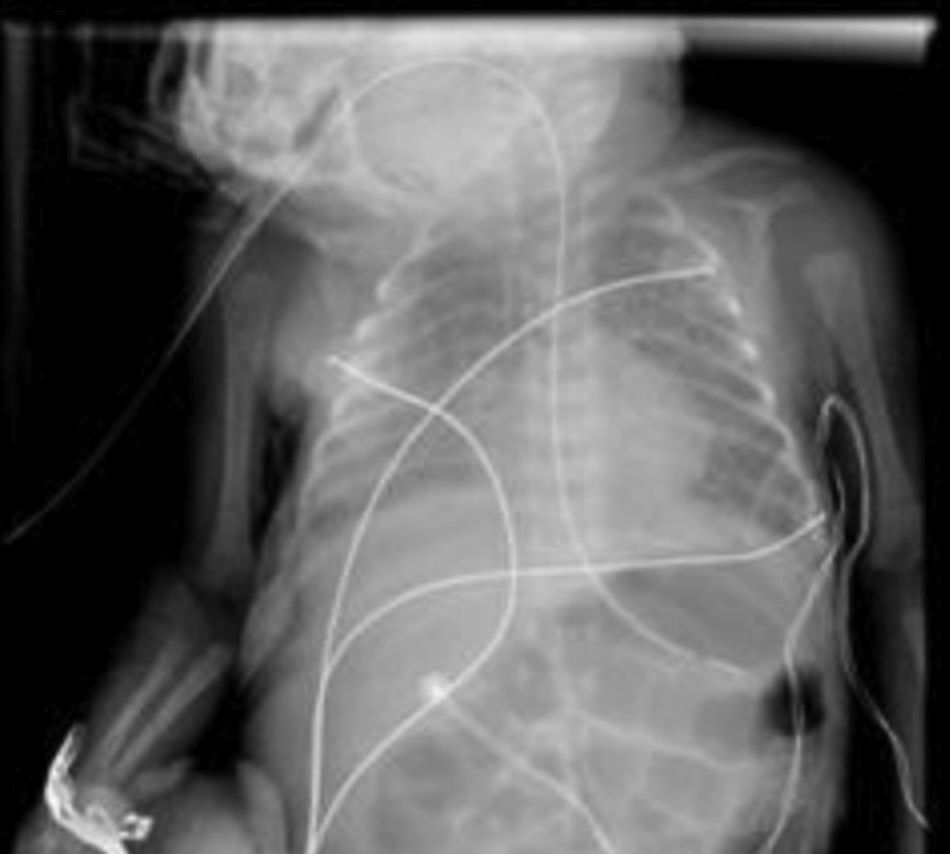
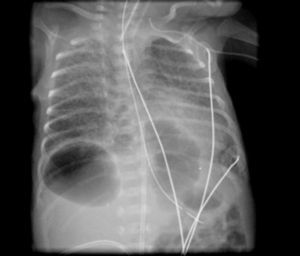
He was intubated after birth and received synchronized conventional mechanical ventilation from the 2nd minute of life. He needed surfactant administration three times, because of severe respiratory distress syndrome (RDS). On day (D) two of life he presented a pulmonary hemorrhage. On D17 he was moved from SIPPV+ volume guarantee (maximum settings: InspP=24cmH2O; frequency=60min–1; PEEP=5cmH2O; FiO2=0.6; VG=6.5ml/kg) to high frequency oscillatory ventilation (maximum settings: MAP=18; DeltaP=38; iT=33%; Freq=15Hz; FiO2=1). Blood cultures were negative as was reactive C protein. A 10 days course of systemic dexamethasone was started. Large cystic pneumatoceles appeared in the right and left lower lobe on D19 (Figs. 1 and 2). Taking a wait and see attitude, the pneumotoceles spontaneously regressed on D32 (Fig. 3).2 Serial cultures of tracheal aspirates from D15 became positive for an extended-spectrum beta-lactamase (ESBL) Klebsiella pneumonia strain, only sensitive to amikacin.
Overall, during the neonatal intensive care unit admission the baby was under mechanical ventilation for 90 days, suffered from two episodes of hypertensive pneumothorax (D14 and D25), two episodes of nosocomial sepsis without isolation of agent on blood cultures (D48 and D80) treated with vancomycin+cefotaxime+amikacin, underwent one surgery for retinopathy of prematurity (D68) and presented one episode of necrotizing enterocolitis (D48) treated with vancomycin+cefotaxime+amikacin+metronidazole, for 10 days. Serial blood cultures were negative. Bronchial aspirates were positive an ESBL-Klebsiella pneumonia from D15 to D96, despite two cycles of treatment with systemic amikacin. He died on D96.
Large cystic pneumatoceles are rare in advanced BPD.1 They are a manifestation of intrathoracic air-leaks of prematurity and are markers for ventilator-induced lung injury and are associated with significant mortality similar to other intrathoracic air-leaks.2 Pneumatoceles may also occur following acute pneumonia, commonly caused by Staphylococcus aureus in children.5 Limited data are available about infective pulmonary cysts in newborns. A case of a newborn, who developed multiple pneumatoceles after Escherichia coli pneumonia has been described.5 The case of a premature infant with a kanamycin resistant klebsiella pneumonia in the first week of life is reported in the literature.6 In infants with pneumatoceles, positive endotracheal culture is a frequent finding and correlates with persistence.7 Infections caused by multidrug-resistant Gram negative bacilli that produce ESBL enzymes have been reported with increasing frequency in intensive-care units and are associated with significant morbidity and mortality.8
Most pneumatoceles disappear spontaneously and do not cause severe symptoms.2 Pneumatoceles may need percutaneous evacuation under fluoroscopic guidance and/or lobectomy in worsening disease.9,10 If the clinical condition allows, an expectant attitude is advised, since many cases may resolve spontaneously.2
Our patient's course was complicated by significant neonatal comorbidities, and the pneumatoceles appeared despite high frequency oscillatory ventilation and a systemic course of dexamethasone. Although without systemic markers of infection, the ESBL Klebsiella pneumonia colonization of the tracheal tree may have played a role in both the severity of the lung disease and its evolution to chronicity. Since the disease did not worsen, close expectant observation was enough and the pneumatoceles spontaneously regressed.
With the presentation of this clinical report on a preterm newborn the authors want to highlight the natural history of pneumatoceles with spontaneous regression and its association to a ESBL Klebsiella pneumonia species in tracheal aspirates.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.